This article introduces the paper 'A Review on Anodizing Process of Aluminum and Non-Aluminium Alloys' published by 'ResearchGate'.
1. Overview:
- Title: A Review on Anodizing Process of Aluminum and Non-Aluminium Alloys
- Author: Dr.A.Renuka Prasad and Dr.Shree Prakash
- Publication Year: September 2023
- Publishing Journal/Academic Society: National conference on Recent Advances in Mechanical, Robotics & Technology, Srinivas University, Mukka, Mangalore, June 28-29, 2022.
- Keywords: Anodizing Process, Types of Anodizing Process, degreasing, pickling, Hard Anodizing.
2. Abstracts or Introduction
This paper provides a comprehensive review of the anodizing process applied to aluminum and non-aluminum alloys, a critical surface treatment for enhancing corrosion resistance in industrial applications. Anodizing, achieved by making the workpiece the anode in a suitable electrolytic cell, necessitates meticulous surface pretreatment, primarily involving degreasing and pickling, to ensure a chemically clean surface. Degreasing employs special detergents to eliminate oils, grease, and solid particulates, while pickling utilizes chemical solutions to remove natural oxides and surface compounds, thereby promoting surface conductivity for subsequent electrochemical processes like anodizing. The review delves into surface characterization and elucidates the combined effects of surface roughness, pre-treatments (degreasing and pickling), and anodization on the fatigue life of alloys.
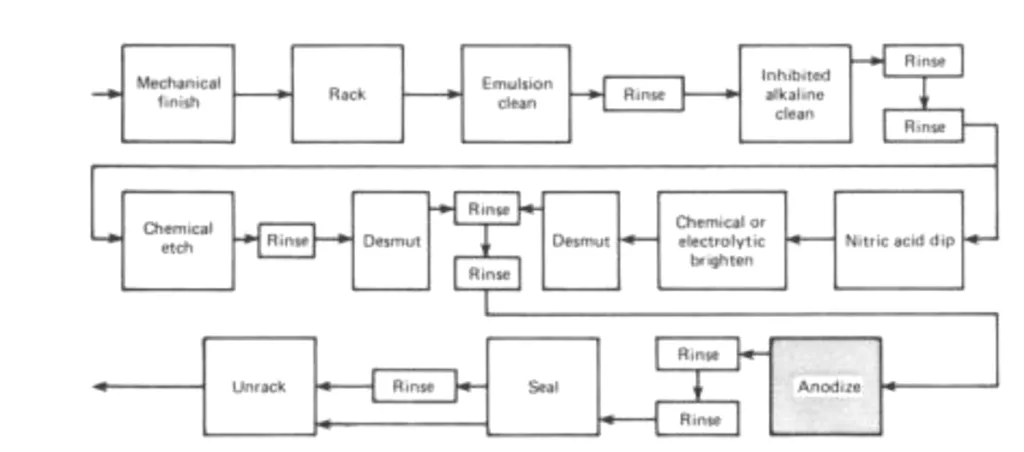
The introduction elaborates on anodizing as a conversion coating process, transforming the aluminum surface and its alloys into porous aluminum oxide. Distinguished from electroplating where the workpiece acts as a cathode, anodizing leverages the aluminum part as the anode in an electrolytic cell [2]. While predominantly associated with aluminum, analogous processes extend to other base metals such as magnesium, titanium, and zinc. The scope of this review is confined to aluminum and its alloys, acknowledging the versatility of anodizing aluminum across diverse electrolytes and operating conditions, encompassing electrolyte concentration and composition, additives, temperature, and voltage. The paper identifies three principal anodizing processes: chromic acid anodizing, sulfuric acid anodizing, and hard anodizing, alongside less common processes employing sulfuric acid with additives like oxalic acid or boric acid [1,4]. It is noted that typical anodic coatings, excluding thicker hard anodized coatings, range from 5 to 18 μm (0.2 to 0.7 mil) in thickness. The operational sequence from surface preparation to sealing in anodizing is visually represented in Fig. 1.
3. Research Background:
Background of the Research Topic:
The research addresses the critical need for enhanced corrosion resistance in aluminum alloys, a material widely utilized in various industries. Anodizing is established as a pivotal surface treatment technique employed industrially to achieve this enhancement. This process leverages electrochemical principles to convert the metallic surface into a durable, corrosion-resistant oxide layer.
Status of Existing Research:
Anodizing technology is well-established, with various types of processes tailored to specific applications and alloy systems. Existing research encompasses different anodizing methods, including chromic acid, sulfuric acid, and hard anodizing, each with distinct electrolytes, operating parameters, and resultant coating properties. Surface preparation techniques like degreasing and pickling are recognized as essential prerequisites for successful anodizing.
Necessity of the Research:
Despite the maturity of anodizing technology, a comprehensive understanding of the interplay between surface preparation, process parameters, and resultant material properties, particularly fatigue life, remains crucial. This review is necessitated by the ongoing demand for optimized anodizing processes that not only enhance corrosion resistance but also maintain or improve the mechanical integrity of the treated components. Specifically, elucidating the "coupled effects of surface roughness and pre-treatments, degreasing and pickling, along with anodization on fatigue life of alloys" is essential for advancing the application of anodizing in critical engineering components.
4. Research Purpose and Research Questions:
Research Purpose:
The primary research purpose is to conduct a review focused on "surface characterization and to demonstrate the coupled effects of surface roughness and pre-treatments, degreasing and pickling, along with anodization on fatigue life of alloys." This review aims to consolidate existing knowledge and provide a handbook-level understanding of these interconnected aspects of the anodizing process.
Key Research:
The key research areas investigated in this review are:
- Surface characterization techniques relevant to anodized aluminum and non-aluminum alloys.
- The effects of pre-treatment processes, specifically degreasing and pickling, on the surface condition prior to anodizing.
- The influence of anodizing process parameters on surface roughness and coating characteristics.
- The relationship between anodizing, pre-treatments, surface roughness, and the resultant fatigue life of alloys.
Research Hypotheses:
While not explicitly stated as formal hypotheses, the research implicitly operates under the premise that:
- Surface preparation significantly impacts the quality and performance of anodic coatings.
- Different anodizing processes and parameters yield varying surface characteristics and corrosion resistance.
- Anodizing, in conjunction with pre-treatments, has a demonstrable effect on the fatigue life of aluminum and non-aluminum alloys.
5. Research Methodology
Research Design:
This study employs a review-based research design. It synthesizes and analyzes existing literature and established knowledge pertaining to the anodizing process.
Data Collection Method:
The data collection method is based on a comprehensive review of published literature, including academic papers, industry handbooks, and technical reports, focusing on anodizing processes for aluminum and non-aluminum alloys.
Analysis Method:
The analysis method involves a descriptive and comparative synthesis of the collected literature. The review systematically categorizes and summarizes information related to surface preparation, different types of anodizing processes (chromic acid, sulfuric acid, hard anodizing), equipment requirements, advantages, and limitations. The analysis aims to present a structured overview of the anodizing process at a handbook level.
Research Subjects and Scope:
The research subject is the anodizing process for both aluminum and non-aluminum alloys. The scope encompasses:
- Surface preparation techniques (degreasing, pickling, etching, desmutting).
- Detailed descriptions of chromic acid, sulfuric acid, and hard anodizing processes.
- Equipment requirements for various anodizing processes.
- Anodizing of non-aluminum materials (magnesium, titanium, zinc).
- Advantages and limitations of the anodizing process.
6. Main Research Results:
Key Research Results:
The review elucidates several key aspects of the anodizing process:
- Surface Preparation: A "chemically clean surface (free of all grease and oil, corrosion products, and the naturally occurring aluminum oxide)" is paramount for successful anodizing. The sequence involves cleaning, etching, pickling, and desmutting. Traditionally, vapor degreasing was employed, but alternatives like "solvent wiping or alkaline soak cleaning" are now prevalent due to environmental regulations. For specular surfaces, brightening treatments are utilized [8].
- Chromic Acid Process: This process is preferred for "components such as riveted or welded assemblies" due to the less corrosive nature of chromic acid compared to sulfuric acid. It yields a "yellow to dark-olive finish" and operates with solutions containing "3 to 10 wt% Cr O3".
- Sulfuric Acid Process: The basic operations are similar to the chromic acid process. However, it is cautioned that "parts or assemblies that contain joints or recesses that could entrap the electrolyte should not be anodized in the sulfuric acid bath [9]." Sulfuric acid concentrations range from "12 to 20 wt%".
- Hard Anodizing Process: Distinguished by operating temperature, addition agents, voltage, and current density, hard anodizing produces "a considerably heavier coating then conventional sulfuric acid anodizing." It typically operates at temperatures from "0 to 10°C (32 to 50°F)" and current densities between "2 and 3.6 A/dm² (20 and 36 A/ft²)."
- Equipment for Anodizing: The review details specific equipment requirements for chromic acid and sulfuric acid anodizing, including tank materials, cathode considerations, temperature control, and masking techniques. For chromic acid, "Low-carbon steel tanks are satisfactory," while sulfuric acid anodizing may utilize "low-carbon steel lined throughout with plasticized polyvinyl chloride."
- Anodizing of Non-Aluminum Materials: The paper extends to magnesium, titanium, and zinc anodizing, outlining process variations and specific applications. For magnesium, "Dow 9 process" and "HAE and Dow 17 processes" are discussed. Titanium anodizing is used to "impart properties other than corrosion resistance," such as galling resistance. Zinc anodizing provides "decorative, yet protective, coatings."
- Advantages of Anodizing: The review highlights several advantages, including "Increase corrosion resistance," "Improve decorative appearance," "Increase abrasion resistance," "Improve adhesive bonding," "Improve lubricity," and "Provide unique, decorative colors."
- Anodizing Process Limitations: Limitations are attributed to "Composition of the aluminum alloy, surface finish, prior processing, temper or heat treatment." For instance, "The chromic acid process should not be used to anodize aluminum casting alloys containing more than 5% Cu or more than 7.5% total alloying elements."
Analysis of presented data:
The paper synthesizes a substantial body of knowledge on anodizing, presenting a structured overview of process parameters, equipment, and material considerations. Figure 1 visually summarizes the "Sequence for Anodizing Process," illustrating the steps from "Mechanical finish" to "Seal." The review emphasizes the importance of process control and material selection in achieving desired anodic coating properties.
Figure Name List:
- Fig.1: Sequence for Anodizing Process.
7. Conclusion:
Summary of Key Findings:
This review underscores the significance of anodizing as a critical corrosion protection method for high strength aluminum alloys, which are extensively used due to their favorable weight-specific mechanical properties. The alloying elements that enhance strength concurrently increase susceptibility to corrosion, necessitating robust protection systems. Anodic oxide layers serve this essential function by providing a barrier against corrosive electrolytes. The complexity of anodizing is highlighted, encompassing process parameters (voltage, temperature), electrolyte nature, substrate material, and pre- and post-anodizing treatments. The review emphasizes that anodizing should be considered within a broader process context rather than as an isolated step. Anodizing enhances wear resistance, corrosion resistance, abrasion resistance, and lubricity of cast aluminum alloys.
Academic Significance of the Study:
This study provides a valuable academic contribution by consolidating and structuring the extensive knowledge base surrounding aluminum and non-aluminum alloy anodizing. It serves as a comprehensive handbook-level resource for researchers, engineers, and students seeking a detailed understanding of anodizing principles, processes, and applications.
Practical Implications:
The practical implications of this review are substantial for industrial applications of anodizing. It offers guidance for process selection, optimization, and troubleshooting, enabling practitioners to achieve desired coating properties and performance. The detailed discussion of process parameters, equipment, and limitations provides actionable insights for improving anodizing operations in manufacturing settings.
Limitations of the Study and Areas for Future Research:
As a review paper, the limitations are inherent in the scope of the reviewed literature. While providing a broad overview, it may not delve into the most recent advancements or highly specialized applications in extreme detail. Areas for future research could include:
- In-depth investigations into the fatigue life performance of anodized alloys under specific service conditions.
- Development of novel electrolytes and process parameters for enhanced coating properties and efficiency.
- Exploration of advanced surface characterization techniques for anodic coatings.
- Studies focused on minimizing the environmental impact of anodizing processes.
8. References:
- K. C. Ludema, “Introduction to wear,” in Friction, Lubrication, and Wear Technology, vol. 18 of The ASM Handbook, pp. 320–628, 1992.
- S.Mischler and P. Ponthiaux, “A round robin on combined electrochemical and friction tests on alumina/stainless steel contacts in sulphuric acid,” Wear, vol. 248, no. 1-2,pp. 211–225, 2001.
- S. Mischler and P. Ponthiaux, “Analytical solution for precessional magnetization switching in exchange biased high perpendicular anisotropy nanostructures,” Journal of Physics D: Applied Physics, vol. 39, 7 pages, 2006.
- S.W.Watson,F. J.Friedersdorf, B.W.Madsen, andS. D. Cramer, “Methods of measuring wear-corrosion synergism,” Wear, vol. 181–183, no. 2, pp. 476–484, 1995.
- Z. Abdel Hamid and M. T. Abou Elkhair, “Development of electro less nickel-phosphorous composite deposits for wear resistance of 6061 aluminum alloy,” Materials Letters, vol. 57, no.3, pp. 720-726, 2002.
- D. Landolt, “Electrochemical and materials aspects of tribo corrosion systems,” in Corrosion and Surface Chemistry of Metals, pp. 227–274, EPFL Press, Lausanne, Switzerland, 2006.
- I. J. Polmear, Light Alloys: Metallurgy of the Light Metals, Butterworth- Heinemann, Arnold, Mo, USA, 1995.
- R. Bosch, Bosch Automotive Handbook, Bentley Publishers, Robert Bentley, Cambridge, Mass, USA, 7th edition, 2007.
- P. Marcus, “Introduction to the fundamentals of corrosion,” in Corrosion: Fundamentals, Testing, and Protection, vol. 13 of ASM Handbook, pp. 3–4, ASM International, 2003.
- B. O. Adewuyi, “The influence of Fe variation on the corrosion behavior of heat treated aluminium alloys in tomato juice,” Nigerian Journal of Technology, vol. 21, no. 1, pp. 72–78, 2002.
- X. Sudarshan and M. K. Surappa, “Dry sliding wear of fly ash particle reinforced A356 Al composites," Wear, vol. 265, no. 3-4, pp. 349–360, 2008.
- D.Tabatabai, S. Szillies, F. Feil, G. Grundmeier, and W. F¨urbeth, “Self-healing corrosion protective coatings for magnesium alloys by modifying anodizing layers with corrosion inhibitors," in Proceedings of the Euro corr Abstracts, no. 4575, Stockholm, Sweden, September 2011.
- M. M. Khrushchov, “Principles of abrasive wear,” Wear, vol. 28, pp. 69–88, 1974.
- H. Joseph, “Abrasive wear,” in Friction, Lubrication, and Wear Technology, vol. 18 of The ASM Handbook, pp. 337–341, 1992.
- A. G. Evans, “Impact damage mechanics: solid projectiles,” in Treatise on Materials Science and Technology, vol. 16, pp. 1–67, Academic Press, 1979.
- A.W. Brace, Ed., Hard Anodizing of Aluminum, Technicopy Ltd., 1987.
- A.W. Brace and EG. Sheas by, The Technology of Anodizing Aluminum, 2nd ed., Technicopy Ltd., 1979.
- D. Montgomery, Ed., Light Metals Finishing Process Manual, American Electroplaters and Surface Finishers Society, 1990.
- S. Wemick, R. Pinner, and P.G. Sheasby, The Surface Treatment and Finishing of Aluminum and Its Alloys, 5th ed., Finishing Publications Ltd., 1987.
- O. O. Ajibola and B. O. Jimoh, “Aluminium recycling industries in Nigeria: entrepreneurship challenges and opportunities,” in Proceedings of the 7th Engineering Forum, vol. 2, pp. 238–247, Ado Ekiti, Nigeria, November 2011.
- Shingubara, S.; Morimoto, K.; Sakaue, H.; Takahagi, T. Self-organization of a porous alumina nanohole array using a sulfuric/oxalic acid mixture as electrolyte. Electrochem. Solid-State Lett. 2004.
- Savas, T.P.; Earthman, J.C. Surface characterization of 7075-T73 aluminum exposed to anodizing pretreatment solutions. J. Mater. Eng. Perform. 2008.
9. Copyright:
- This material is "Dr.A.Renuka Prasad and Dr.Shree Prakash"'s paper: Based on "A Review on Anodizing Process of Aluminum and Non-Aluminium Alloys".
- Paper Source: https://www.researchgate.net/publication/374005576
This material was summarized based on the above paper, and unauthorized use for commercial purposes is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.