Boost Aluminum Die Casting Performance: How Cerium Additions Conquer Corrosion from Copper Impurities
This technical summary is based on the academic paper "Surface Corrosion Response of Al Alloys A383 and Aural 2 with Ce Additions in Aqueous NaCl and Salt-fog Environments" by Michael James Thompson, published as a Master's Thesis at the University of Tennessee, Knoxville (2023).
Keywords
- Primary Keyword: Cerium in Aluminum Alloys
- Secondary Keywords: Aluminum corrosion resistance, A383 corrosion, Aural 2 alloy, recycled aluminum die casting, copper impurity in aluminum, salt fog testing, HPDC material science, intermetallic compounds.
Executive Summary
- The Challenge: Using cost-effective recycled aluminum in die casting is hampered by high copper (Cu) content, which severely degrades the corrosion resistance of the final components.
- The Method: Researchers introduced varying amounts of Cerium (Ce) into two common Al-Si die-casting alloys (A383 and Aural 2) with elevated copper levels and subjected them to rigorous electrochemical and salt-fog corrosion tests.
- The Key Breakthrough: Adding Cerium up to 1.5 wt.% significantly improves corrosion resistance by binding detrimental copper and iron impurities into stable, less reactive intermetallic compounds.
- The Bottom Line: Cerium acts as a powerful "scavenger" for impurities, enabling the use of more recycled aluminum feedstock in high-performance applications without compromising component longevity in corrosive environments.
The Challenge: Why This Research Matters for HPDC Professionals
In the aluminum die casting industry, the push for sustainability and cost reduction has made recycled (secondary) aluminum an attractive feedstock. Over 70% of aluminum used in the US comes from recycled sources. However, this material often contains a high concentration of impurities, particularly copper. While copper is a useful strengthening agent, it is notoriously detrimental to corrosion resistance. It forms galvanic cells within the alloy's microstructure, creating pathways for corrosion, especially along grain boundaries.
To meet strict performance specifications, manufacturers are often forced to dilute this recycled feedstock with expensive, energy-intensive primary aluminum. This practice negates many of the cost and environmental benefits of recycling. This research tackles this critical industry challenge head-on by exploring a novel alloying addition—Cerium (Ce)—to improve the impurity tolerance of standard die-casting alloys, paving the way for greater use of recycled materials.
The Approach: Unpacking the Methodology
To validate the effects of Cerium, the research team conducted a systematic experimental study grounded in real-world die-casting conditions.
Method 1: Alloy Formulation and Casting
- Base Materials: Two common Al-Si die-casting alloys were used: A383 (which has a higher tolerance for impurities) and Aural 2 (A2, which has tighter compositional controls).
- Alloying Additions: A baseline of 0.6 wt.% copper was added to simulate a recycled feedstock. Then, varying amounts of Cerium (from 0.5 wt.% to 2.5 wt.%) were systematically introduced into different batches.
- Casting Process: The alloys were melted and cast into a water-cooled copper mold to simulate the rapid solidification rates typical of the high-pressure die casting (HPDC) process, ensuring the resulting microstructure was industrially relevant.
Method 2: Corrosion Performance Testing
- Electrochemical Testing: Potentiodynamic polarization (PDP) tests were performed in a 0.1 M NaCl (salt) solution. This technique measures key parameters like corrosion current (Icorr) and corrosion potential (Ecorr) to provide a quantitative corrosion rate.
- Accelerated Environmental Testing: Samples were exposed to a 5% NaCl salt fog environment for 1800 hours, following the ASTM B117 standard. This long-duration test mimics the harsh conditions automotive components face from road salt, allowing for the evaluation of pitting and overall surface degradation.
Method 3: Microstructural Characterization
- Analysis Techniques: The researchers used Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDS), and X-ray Diffraction (XRD) to analyze the alloys.
- Objective: These methods allowed for the identification of the specific phases and intermetallic compounds present in the as-cast alloys, as well as the corrosion products that formed after testing, revealing the underlying mechanism of Ce's protective effect.
The Breakthrough: Key Findings & Data
The study produced clear, data-driven evidence of Cerium's benefits, identifying an optimal concentration for maximizing corrosion resistance.
Finding 1: Cerium Additions Dramatically Reduce Corrosion Rate
The addition of Cerium had a profound impact on the corrosion performance of both alloys. In the PDP tests, the corrosion rate is directly related to the corrosion current (Icorr).
As shown in Table 4 of the paper, the addition of 1.5 wt.% Ce to the A383 alloy (with 0.6 wt.% Cu) reduced the corrosion rate from 0.71 mpy (miles per year) in the base alloy to 0.38 mpy, a reduction of 46%. For the more sensitive A2 alloy, the corrosion rate dropped from 0.09 mpy to 0.05 mpy, a 44% improvement. This demonstrates that Ce is effective at mitigating the corrosive effects of copper.
Finding 2: An Optimal Cerium Level of 1.5 wt.% Was Identified
The research revealed that the benefits of Cerium are not limitless. The most significant improvement in corrosion resistance for both alloys was achieved at an addition level of 1.5 wt.% Ce.
According to the data in Table 4, increasing the Ce content to 2.0 wt.% and 2.5 wt.% yielded no further benefit and, in fact, caused a slight increase in the corrosion rate. For A383, the rate increased back to 0.67 mpy at 2.0 wt.% Ce. The authors suggest this may be due to the formation of a higher fraction of needle-shaped Ce-containing phases, which increases the number of interfaces in the microstructure that can act as potential sites for corrosion. This finding is critical for cost-effective implementation, as it defines a clear target for optimal performance without wasting an expensive alloying element.
Practical Implications for R&D and Operations
- For Process Engineers & Metallurgists: This study suggests that adding approximately 1.5 wt.% Cerium to the melt can significantly improve the corrosion resistance of Al-Si castings made from secondary aluminum with elevated copper content. This provides a new tool for alloy design and for meeting stringent customer specifications.
- For Quality Control Teams: The data in Figure 11 of the paper, which shows a dramatic reduction in maximum corrosion pit depth with Ce additions, is particularly noteworthy. For A383, the pit depth decreased from 1478 µm to 511 µm with 1.5 wt.% Ce. This could inform new, more robust quality inspection criteria for components intended for corrosive service environments.
- For Procurement & Sourcing Specialists: The findings provide a strong technical basis for increasing the use of lower-cost, secondary (recycled) aluminum feedstock. By managing impurities with Cerium, companies can potentially reduce raw material costs and improve the sustainability of their operations without sacrificing product quality.
Paper Details
Surface Corrosion Response of Al Alloys A383 and Aural 2 with Ce Additions in Aqueous NaCl and Salt-fog Environments
1. Overview:
- Title: Surface Corrosion Response of Al Alloys A383 and Aural 2 with Ce Additions in Aqueous NaCl and Salt-fog Environments
- Author: Michael James Thompson
- Year of publication: 2023
- Journal/academic society of publication: Master's Thesis, University of Tennessee, Knoxville
- Keywords: Aluminum alloys, A383, Aural 2, Cerium, Corrosion, NaCl, Salt-fog, Copper impurity, Recycling, Intermetallics
2. Abstract:
Copper is commonly used in aluminum alloys to increase its strength by solid solution and precipitation strengthening, however, the corrosion resistance is inversely related to the amount of copper in the alloy. Over 70 percent of material used to produce aluminum alloys in the US come from recycled (secondary) alloys, many of which have a copper content of more than one percent by weight. Alloys with tightly controlled tolerances, where copper is seen as an impurity, are unable to utilize many of the recycling feedstock without adding newly processed (primary) aluminum to dilute impurities to within specifications. Primary aluminum is much more costly to produce due to the series of energy intensive processes required to make aluminum from raw materials such as bauxite. Adding cerium to aluminum alloys can increase the tolerance of impurities such as copper by binding them into more corrosion resistant intermetallic compounds that mitigate copper denuded zones at grain boundaries where most corrosion damage occurs. The corrosion response and microstructural changes of two Al-Si die-casting alloys in NaCl salt solution were investigated in the base alloys and after adding 0.6 weight percent copper and cerium additions up to 2.5 weight percent. Corrosion resistance was directly related to cerium additions, with the most significant improvement at 1.5 weight percent cerium. Further cerium additions yielded no further benefit to corrosion resistance. Compositional and structural characterization methods were used to determine the corrosion products that formed during exposure to NaCl salt solution. The majority of corrosion damage was observed at the interface of Al(FeMn)Si based intermetallics and the Al-matrix.
3. Introduction:
Aluminum (Al) alloys are a primary material choice for automotive components due to their strength-to-weight ratio and corrosion resistance. Al production involves primary Al from bauxite and secondary Al from recycled scrap. Secondary Al is ~90% more energy efficient but often contains impurities like copper (Cu) that exceed the tolerances for many high-performance alloys. Cu-rich phases form galvanic cells that accelerate corrosion, particularly at grain boundaries. To use secondary Al, foundries must often dilute it with expensive primary Al. This research investigates the use of Cerium (Ce) as an alloying addition. Ce has been shown to be a scavenging agent that can form stable intermetallic compounds with impurities such as Fe and Cu. This study aims to demonstrate that the addition of Ce to commercial Al alloys can increase their corrosion resistance, thereby increasing their tolerance for impurity elements and broadening the viability of recycled Al feedstock.
4. Summary of the study:
Background of the research topic:
The use of recycled (secondary) aluminum in die casting is economically and environmentally advantageous. However, the accumulation of impurities, particularly copper, degrades corrosion performance, limiting the application of these materials and necessitating the use of costly, energy-intensive primary aluminum for dilution.
Status of previous research:
Prior literature has established the detrimental effect of copper on the corrosion resistance of aluminum alloys. Research has also shown that rare-earth elements, such as Cerium, can act as "gettering" or scavenging agents for impurities like iron and copper by forming thermodynamically stable intermetallic compounds, which could improve corrosion performance.
Purpose of the study:
The purpose of this work was to investigate the influence of intentional additions of copper and cerium on the microstructure and corrosion response of two commercial Al-Si die-casting alloys, A383 and Aural 2 (A2). The study aimed to determine if Ce additions could mitigate the negative corrosion effects of Cu, thereby improving the alloys' tolerance for impurities found in recycled feedstock.
Core study:
The study involved casting A383 and A2 alloys with a 0.6 wt.% addition of Cu and systematic additions of Ce ranging from 0.5 to 2.5 wt.%. The corrosion behavior of these alloys was evaluated using potentiodynamic polarization (PDP) in an aqueous NaCl solution and through a 1800-hour salt-fog exposure test (ASTM B117). The as-cast microstructures and post-corrosion surfaces were characterized using XRD, SEM, and EDS to identify the phases present, the corrosion products formed, and the mechanisms of corrosion.
5. Research Methodology
Research Design:
The study employed an experimental design to compare the corrosion performance of two base alloys (A383 and A2) against modified compositions. The independent variables were the additions of Cu (0.6 wt.%) and Ce (0, 0.5, 1.0, 1.5, 2.0, and 2.5 wt.%). The dependent variables were corrosion rate, corrosion potential, and corrosion morphology.
Data Collection and Analysis Methods:
Alloys were prepared by melting base materials in an induction furnace and adding pure Cu and Ce. The melts were cast into a water-cooled copper mold to achieve a rapid solidification rate comparable to die casting. Corrosion behavior was quantified using a Gamry Instruments Potentiostat for PDP tests. Long-term corrosion was assessed in a Model 22 Auto Technology salt fog cabinet. Microstructural analysis was performed using an FEI Teneo LoVac SEM, and phase identification was conducted with a Malvern Panalytical Empyrean diffractometer.
Research Topics and Scope:
The research focused on the surface corrosion response of Al-Si die-casting alloys. The scope encompassed the effects of Cu and Ce additions on microstructure, electrochemical corrosion in NaCl solution, and atmospheric corrosion in a salt-fog environment. The study also investigated the formation mechanisms of corrosion products.
6. Key Results:
Key Results:
- Both alloys showed good castability. The A2 alloy exhibited higher intrinsic corrosion resistance compared to A383, which was attributed to its lower base Cu content.
- Ce additions up to 1.5 wt.% resulted in a decrease in the corrosion rate for both A383 and A2 alloys containing 0.6 wt.% added Cu.
- Further Ce additions beyond 1.5 wt.%, up to 2.5 wt.%, did not provide additional improvement in corrosion resistance and, in some cases, slightly increased the corrosion rate.
- Ce was observed to be highly reactive with impurity elements such as Cu and Fe, leading to the formation of Ce-rich intermetallic structures. This "gettering" effect mitigates the negative impact of these impurities on the alloy's corrosion performance.
- The interfaces of Al(FeMn)Si and Ce-rich phases were identified as being sensitive to corrosion initiation. However, Ce offers better protection against intergranular corrosion compared to phases present without Ce.
- Exposure to the NaCl salt solution resulted in the formation of several corrosion products, identified by XRD as Al2O5Si, Al2O3, SiO2, Cu2O, and Al(OH)3.
Figure Name List:
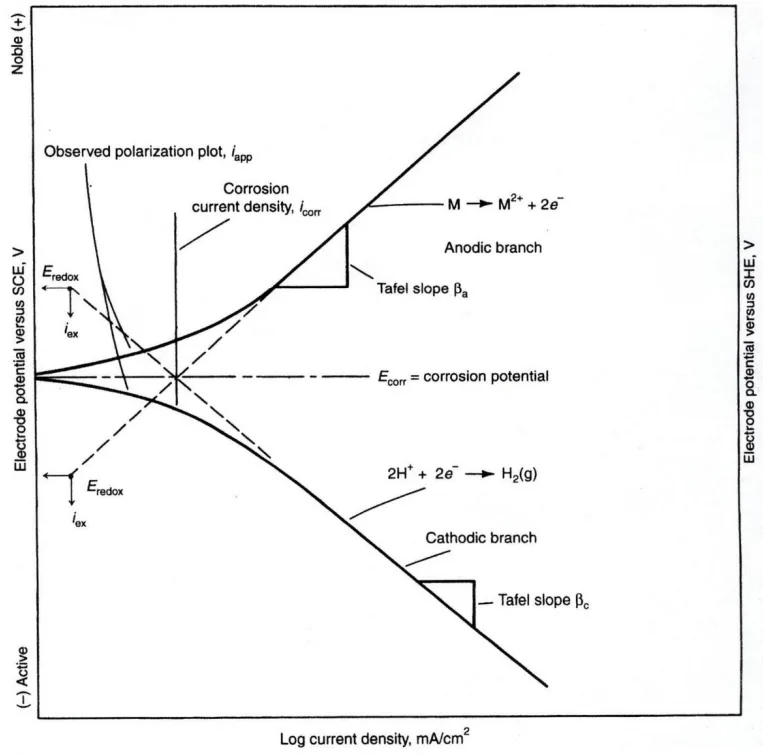
- Figure 1: Schematic of a Tafel plot identifying anodic and cathodic branches, and values for corrosion potential (OCP) and corrosion current density.
- Figure 2: XRD scans of A383, A383+0.6Cu+2.5Ce, A2, A2+0.6Cu+2.5Ce shown a log scale stacked plot.
- Figure 3: BSE micrographs depicting the distribution of phases in (a) A383, (b) A383+0.6Cu, (c)A383+0.6Cu+1.5Ce and (d) A383+0.6Cu+2.5Ce. An increase in the amount of Cu2Al can be seen after the addition Cu is introduced, and the bright phase is due to Ce additions.
- Figure 4: BSE micrographs showing the distribution of phases in (a) A2, (b) A2+0.6Cu, (c) A2+0.6Cu+1.5Ce and (d) A2+0.6Cu2.5Ce. The addition of Cu was mostly found in the Al(FeMn)Si intermetallics rather than Cu2Al. The bright intermetallics were much less prevalent in A2 compared to A383.
- Figure 5: XRD scans of A383 and A383+0.6Cu+2.5Ce after corrosion showing the peaks of oxides and hydroxides including Al2O5Si, Al2O3, SiO2, Cu2O, and Al(OH)3. Selected areas are magnified to help with oxide identification.
- Figure 6: XRD scans of A2 and A2+0.6Cu+2.5Ce showing peaks of major uncorroded phases and oxides present. Selected areas are magnified to help with oxide identification.
- Figure 7: Tafel curves for A383 and A2 depicting the cathodic and anodic branches along with the relative Ecorr and Icorr values. The lower Icorr value and raised shape of the anodic branch of A2 shows the alloy’s higher resistance to corrosion compared to A383.
- Figure 8: Potentiodynamic curves for alloys (a) A383 with Cu and Ce additions and (b) A2 with Cu and Ce additions, and measured Ecorr, Icorr, and corrosion rate for (c) A383 with Cu and Ce additions and (d) A2 with Cu and Ce additions. Corrosion rate decreased in both alloys with Ce additions up to 1.5 wt.% Ce after Cu was added. Further additions of Ce caused the corrosion rate to increase.
- Figure 9: BSE micrographs of corroded samples (a) A383, (b) A383+0.6Cu, (c) A383+0.6Cu+1.5Ce, and (d) A383+0.6Cu+2.5Ce after salt fog exposure for 1800 hrs showing oxides and corrosion pits resulting from the experiment.
- Figure 10: BSE micrographs of corroded samples (a) A2, (b) A2+0.6Cu, (c) A2+0.6Cu+1.5Ce, and (d) A2+0.6Cu+2.5Ce after salt fog exposure for 1800 hrs showing oxides and corrosion pits resulting from the experiment.
- Figure 11: Maximum measured corrosion depths for all tested samples from SEM micrographs. The blue and pink bars correspond to A383 and A2 samples respectively.
- Figure 12: Schematic diagram representing corrosion phenomena including formation of corrosion products and local cell formation at (a) Si-rich segregated regions, (b) interfaces of Ce-rich phases and Al-matrix, (c) corrosion response at the interface of Al matrix and Al(FeMn)Si (d) BSE micrographs of A383+0.6Cu+2.5Ce and (e)BSE micrographs of A2+0.6Cu+2.5Ce.
7. Conclusion:
Two Al-Si based die-casting alloys with and without Cu and Ce additions beyond the base alloy composition were cast to produce a microstructure comparable to die casting. The alloys were subjected to PDP testing and salt fog exposure to determine the corrosion response. From this work, we conclude:
1. Both alloys show good castability, however, A2 showed higher corrosion resistance compared to A383 due to its lower Cu content.
2. Both alloys consist of α-Al (Al-Si alloy), segregated Si, and varieties of aluminides including Al(FeMn)Si, Al2Cu, and AlCeSi. The formation of these aluminides causes segregation of other elements including Cu, Mg, and Si to the interdendritic regions which increase corrosion sensitivity.
3. Ce is highly reactive with elements such as Cu and Fe which can lead to "gettering" these impurities and promoting corrosion resistant intermetallic structures. This mitigates their negative effects on corrosion performance of the alloy. The suppressed activity of Cu leads to a decrease in values for Icorr and corrosion rate.
4. Ce addition up to 1.5 wt. % causes a decrease in the corrosion rate for both alloys; while further additions up to 2.5 wt. % causes the corrosion mode to change from intergranular to planar.
5. The interface of the Al(FeMn)Si and Ce-rich phases are sensitive to corrosion resulting in localized pits and corrosion product formation at their interfaces, however, Ce offers better protection against intergranular corrosion.
6. Exposure to NaCl salt solution causes oxide formation including Al2O5Si, Al2O3, SiO2, Cu2O, and Al(OH)3 as corrosion products.
7. The addition of the Ce is an effective alloying strategy for the design of corrosion resistant Al-alloys that can be recycled.
8. References:
- [1] S. K. Das, J. A. S. Green, and J. G. Kaufman, “The development of recycle-friendly automotive aluminum alloys,” JOM, vol. 59, no. 11, pp. 47–51, Nov. 2007, doi: 10.1007/s11837-007-0140-2.
- [2] R. S. Long, E. Boettcher, and D. Crawford, “Current and Future Uses of Aluminum in the Automotive Industry,” JOM, vol. 69, no. 12, pp. 2635–2639, Dec. 2017, doi: 10.1007/s11837-017-2554-9.
- [3] D. Adamović, T. Vujinović, F. Živić, J. Živković, and M. Topalović, “Application of Aluminum and ITS Alloys in the Automotive Industry With Special Emphasis PN Wheel Rims,” JTTTP - J. TRAFFIC Transp. THEORY Pract., vol. 6, no. 2, Oct. 2020, doi: 10.7251/JTTTP2102087A.
- [4] S. Montijo, “What is Aluminum Used for: Automotive Edition,” Kloeckner Metals Corporation, Apr. 28, 2021. https://www.kloecknermetals.com/blog/what-is-aluminum-used-for-automotive-edition/ (accessed Feb. 27, 2023).
- [5] “Mineral commodity summaries 2022,” Reston, VA, Report 2022, 2022. doi: 10.3133/mcs2022.
- [6] “Energy and Exergy Analysis of the Primary Aluminum Production Processes: A Review on Current and Future Sustainability.” https://www.tandfonline.com/doi/epdf/10.1080/08827508.2010.530721?needAccess=true&role=button (accessed Feb. 27, 2023).
- [7] “Secondary Production 101 | The Aluminum Association.” https://www.aluminum.org/secondary-production-101 (accessed Feb. 01, 2023).
- [8] J. Cui and H. J. Roven, “Recycling of automotive aluminum,” Trans. Nonferrous Met. Soc. China, vol. 20, no. 11, pp. 2057–2063, Nov. 2010, doi: 10.1016/S1003-6326(09)60417-9.
- [9] “Corrosion pathways in aluminium alloys | Elsevier Enhanced Reader.” https://reader.elsevier.com/reader/sd/pii/S1003632617600062?token=4D0C329475B4787368ADF678F2BF36E51334239FBE6728318083162A7AFF2A33BA691C1127393038CD8FCDAAA45E0C4&originRegion=us-east-1&originCreation=20230228012014 (accessed Feb. 27, 2023).
- [10] R. Zhang, N. Birbilis, S. Knight, R. Holtz, R. Goswami, and C. Davies, “A Survey of Sensitization in 5xxx Series Aluminum Alloys,” Corrosion, vol. 72, p. 150903122215004, Sep. 2015, doi: 10.5006/1787.
Expert Q&A: Your Top Questions Answered
Q1: Why were the A383 and Aural 2 alloys specifically chosen for this study?
A1: These two alloys represent a valuable comparison for the die-casting industry. A383 is a widely used alloy known for its good castability and higher tolerance for impurities like iron and copper. In contrast, Aural 2 (A2) is designed for applications requiring better properties and thus has much tighter controls on impurities. By testing both, the researchers could evaluate Ce's effectiveness in a common workhorse alloy and a more performance-sensitive one.
Q2: What is the specific mechanism by which Cerium improves corrosion resistance?
A2: The paper explains that Cerium is highly reactive with impurity elements like copper and iron. It acts as a "scavenger" or "gettering" agent, preferentially bonding with these elements to form new, more complex intermetallic compounds (like AlCeSi). These Ce-containing phases are more corrosion-resistant than the detrimental Al2Cu phases that would otherwise form, effectively neutralizing the negative impact of the impurities.
Q3: The study showed corrosion rates increased slightly when Ce was added beyond 1.5 wt.%. Why did this happen?
A3: The author suggests this is likely due to a change in the microstructure. At higher concentrations, Ce tends to form a greater fraction of needle-shaped intermetallic phases. This morphology increases the total area of interfaces between different phases within the alloy. These interfaces can act as initiation sites for corrosion, so having more of them can slightly offset the chemical benefits of the Ce addition.
Q4: How do the results from the short-term electrochemical (PDP) tests compare to the long-term salt fog exposure?
A4: The paper notes that while both tests confirmed the beneficial trend of adding Ce up to 1.5 wt.%, the absolute corrosion rates and depths differed. This is a known phenomenon. The PDP test provides a rapid, averaged corrosion rate under specific conditions. In the long-term salt fog test, as corrosion pits form, the chemical environment inside the pit becomes more acidic and stagnant, leading to a different, more localized corrosion mechanism that can be more aggressive.
Q5: Besides improving corrosion resistance, what other potential benefits could adding Cerium to recycled aluminum alloys offer?
A5: The paper's introduction mentions that Ce-based intermetallics can also provide increased high-temperature strength and stability. While not the focus of this corrosion study, this suggests that Ce additions could offer multiple performance benefits. This makes it a promising alloying element for creating more robust, high-performance alloys from recycled feedstock.
Conclusion: Paving the Way for Higher Quality and Productivity
The challenge of managing copper impurities in recycled aluminum is a significant barrier to cost-effective, sustainable manufacturing. This research demonstrates a powerful solution: the strategic use of Cerium in Aluminum Alloys. By adding just 1.5 wt.% Cerium, die casters can effectively neutralize the corrosive effects of copper, unlocking the full potential of secondary aluminum feedstock. This breakthrough not only enhances component durability but also offers a direct path to reduced material costs and a smaller environmental footprint.
At CASTMAN, we are committed to applying the latest industry research to help our customers achieve higher productivity and quality. If the challenges discussed in this paper align with your operational goals, contact our engineering team to explore how these principles can be implemented in your components.
Copyright Information
This content is a summary and analysis based on the paper "Surface Corrosion Response of Al Alloys A383 and Aural 2 with Ce Additions in Aqueous NaCl and Salt-fog Environments" by "Michael James Thompson".
Source: https://trace.tennessee.edu/utk_gradthes/9239
This material is for informational purposes only. Unauthorized commercial use is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.