This article introduces the paper "Bending Strength of Salt Core Comprised of KCl-NaCl-Na2CO3-K2CO3 Systems" presented at the J. JFS, Vol. 79, No. 4 (2007) pp. 184~191
1. Overview:
- Title: Bending Strength of Salt Core Comprised of KCl-NaCl-Na₂CO₃-K₂CO₃ Systems
- Authors: Jun Yaokawa, Daisuke Miura, Katsunari Oikawa, Koichi Anzai, Youji Yamada, Hiroshi Yoshii
- Publication Year: 2007
- Publishing Journal/Academic Society: J. JFS (Japan Foundry Engineering Society), Vol. 79, No. 4
- Keywords: salt core, expendable core, carbonate, chloride, die casting, strength, deflection, decomposition
2. Research Background:
- Social/Academic Context of the Research Topic: Aluminum alloy die casting is widely used in automotive parts due to its lightweight, high strength, corrosion resistance, and formability. However, die casting of undercut shapes is challenging. Expendable cores are crucial to overcome this limitation and enable the die casting of complex geometries. Water-soluble salt cores are attractive candidates due to their removability.
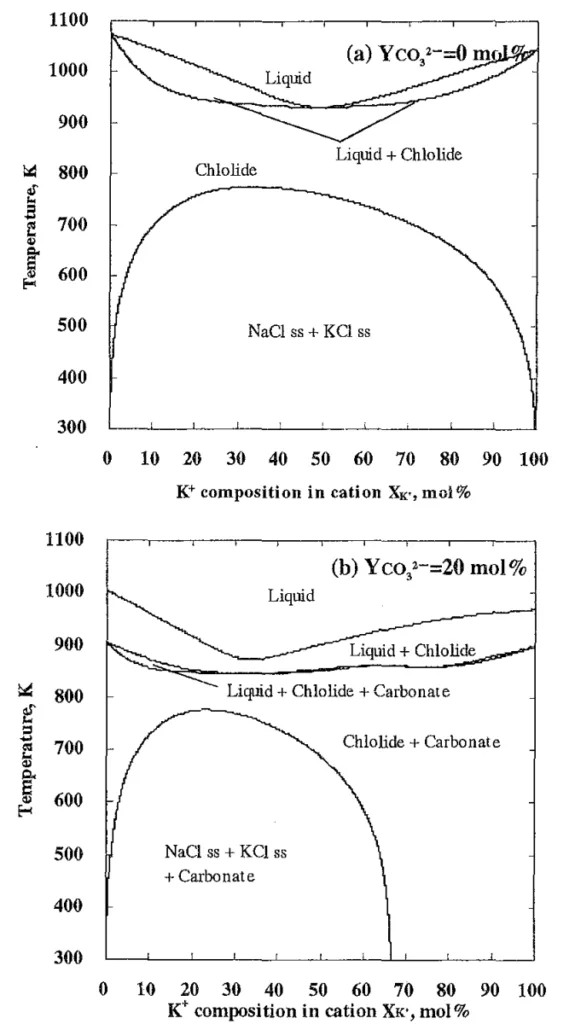
- Limitations of Existing Research: Previous research explored water-soluble salt cores made from binary systems of chlorides and carbonates, such as NaCl-Na₂CO₃ and KCl-K₂CO₃. These systems showed high strength without reinforcement materials, indicating potential for die casting applications. However, for practical application in molten salt core forming, a lower liquidus temperature (873~973K) is desirable. While multi-component systems like KCl-NaCl-K₂CO₃-Na₂CO₃ offer a wide composition range with such low liquidus temperatures, systematic investigations into their strength were lacking.
- Necessity of the Research: To expand the applicability of salt cores in die casting, especially for complex shapes requiring low liquidus temperature and high strength, a detailed investigation of the KCl-NaCl-Na₂CO₃-K₂CO₃ system's strength is necessary. This research aims to address this gap by thoroughly examining the strength characteristics of this multi-component salt system.
3. Research Purpose and Research Questions:
- Research Purpose: To comprehensively investigate the strength of KCl-NaCl-Na₂CO₃-K₂CO₃ salt cores.
- Key Research Questions:
- What are the composition areas within the KCl-NaCl-Na₂CO₃-K₂CO₃ system that exhibit high bending strength?
- How do thermodynamic properties and phase diagrams of this multi-component system relate to the bending strength of salt cores?
- What is the influence of sodium content in the primary chloride phase on the strength of the salt cores?
- What is the relationship between the microstructure and bending strength of salt cores in this system?
- Research Hypotheses:
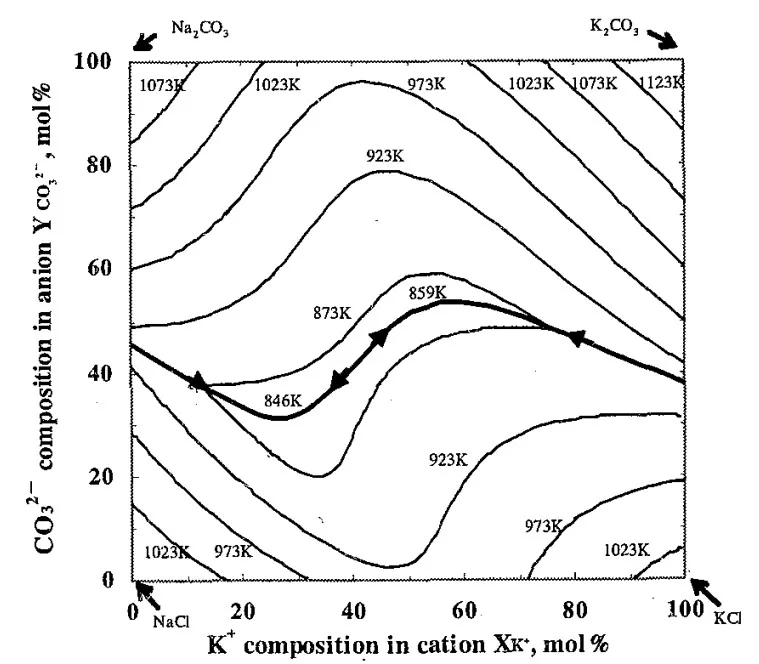
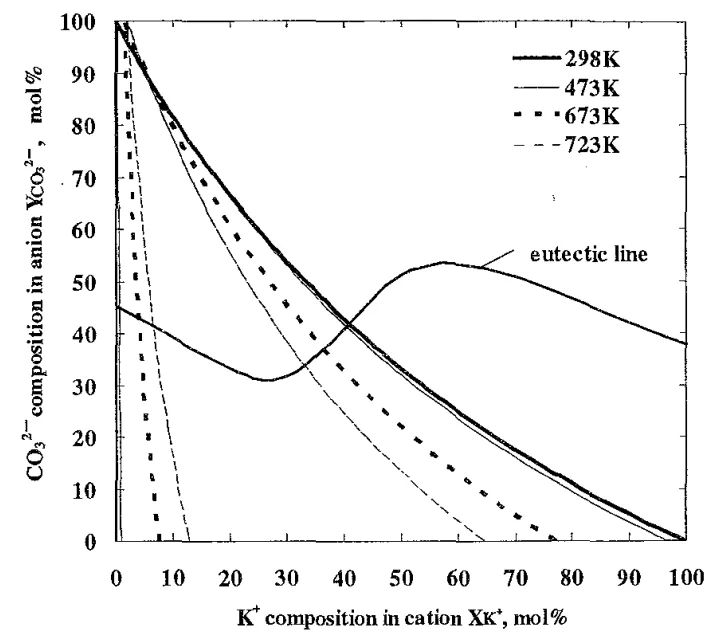
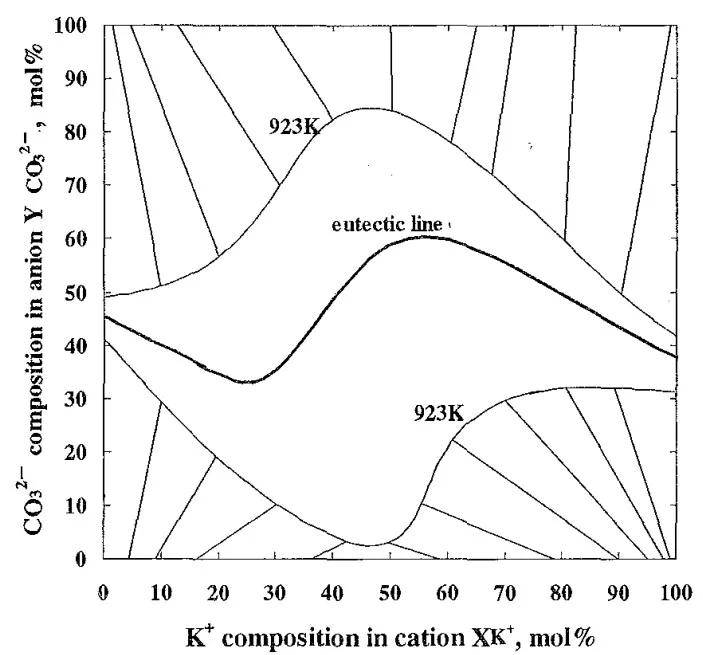
- Based on thermodynamic calculations, specific composition areas within the KCl-NaCl-Na₂CO₃-K₂CO₃ system will exhibit high bending strength due to the coexistence of primary crystals and eutectic structures.
- The strength of the salt cores is influenced by the sodium content in the primary chloride phase.
- Two-phase separation in certain compositions may lead to reduced strength.
4. Research Methodology
- Research Design: Experimental investigation combined with thermodynamic calculations.
- Data Collection Method:
- Four-point bending test: To measure the bending strength of salt core specimens.
- SEM-EDX analysis: To analyze the microstructure and elemental composition of fractured surfaces.
- Analysis Method:
- Thermodynamic calculations: Using Thermo-Calc software and existing thermodynamic data to calculate phase diagrams (liquidus surface, eutectic lines, vertical sections, isothermal sections) and predict composition areas with high strength potential.
- Statistical analysis: Calculating average bending strength and standard deviation from multiple specimens for each composition.
- Microstructural observation: Analyzing SEM micrographs of fractured surfaces to understand the relationship between microstructure and strength.
- Research Subjects and Scope:
- Salt cores made from KCl, NaCl, Na₂CO₃, and K₂CO₃ powders.
- Composition range systematically varied by 10 mol% increments for both cation ratio (XK+) and anion ratio (YCO₃²⁻) to cover the Na⁺-K⁺-Cl⁻-CO₃²⁻ system.
- Focus on bending strength as the primary mechanical property.
5. Main Research Results:
- Key Research Results:
- High Strength Areas: Bending strength exceeding 20 MPa was achieved in three composition areas, and strength over 15 MPa was obtained in another area, although with limited composition range.
- Thermodynamic Prediction Agreement: Experimental results showing high strength areas were in good agreement with theoretical predictions based on thermodynamic data.
- Liquidus Temperature: Liquidus temperatures in high strength areas were between 873K and 973K, suitable for molten salt core fabrication.
- Sodium Content Influence: SEM-EDX analysis revealed that sodium content in the primary chloride phase varied among specimens, and the strength of the primary phase depended on sodium content. Sodium content was identified as a crucial factor in controlling specimen strength.
- Decomposition Region: In the chloride phase decomposition region, strength was relatively low if the primary phase was chloride.
- Statistical/Qualitative Analysis Results:
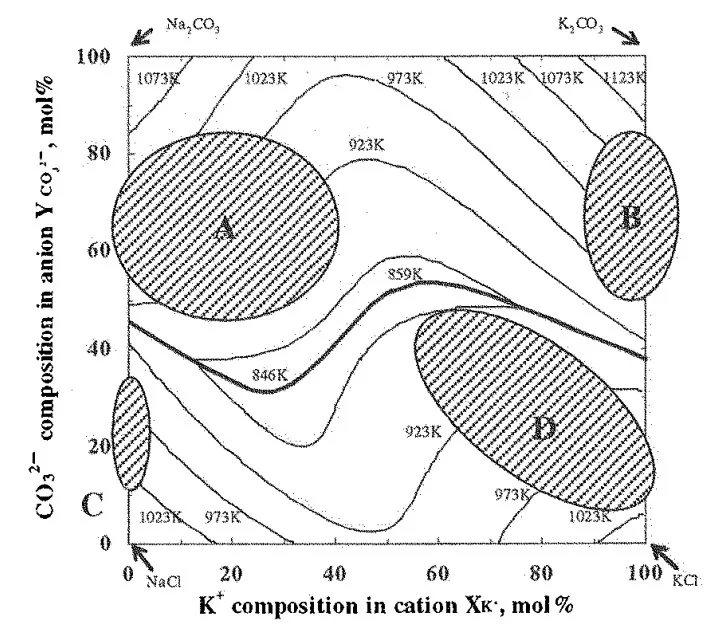
- Strength Map (Fig. 7): A bending strength map (Fig. 7) visually represents the average bending strength across the composition range, highlighting areas with strength above 20MPa, 15-20MPa, and 10-15MPa.
- Defect Observation (Fig. 6): Photographs of specimen cross-sections (Fig. 6) showed internal voids (present in most samples, not strength-related) and surface irregularities/cracks (observed in specific compositions, potentially strength-reducing).
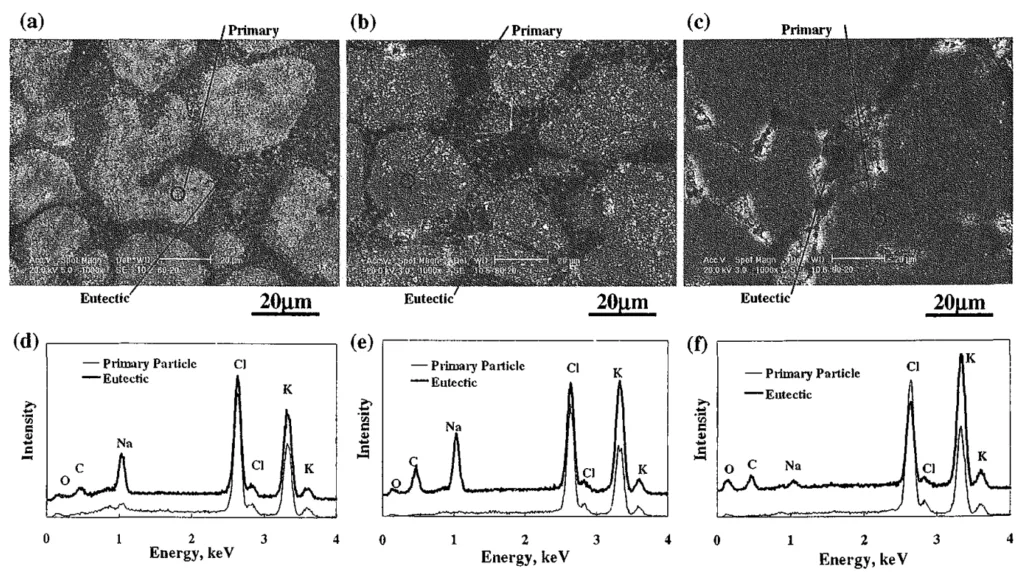
- Microstructure and EDX (Fig. 8 & 9): SEM micrographs (Fig. 8 & 9) and EDX analysis (Fig. 9) of high-strength specimens revealed a microstructure of primary chloride dendrites and eutectic structures. EDX analysis indicated variations in sodium and potassium content within primary chloride and eutectic phases, correlating with strength differences.
- Data Interpretation:
- High strength is associated with compositions where primary crystals and eutectic structures coexist, supporting the deflection mechanism for strength enhancement.
- Sodium content in the primary chloride phase plays a significant role in strength. Optimal sodium content in the primary chloride phase contributes to higher strength.
- Two-phase separation regions generally correspond to lower strength values.
- Figure Name List:
- Fig. 1 Liquidus surface and eutectic line of Na⁺-K⁺-Cl⁻-CO₃²⁻ system calculated with Thermo-Calc¹⁵).
- Fig. 2 Phase diagrams of vertical section calculated with Thermo-Calc¹⁵). (a) CO₃²⁻ composition in anion Yco₃²⁻ = 0 mol% (NaCl-KCl binary system). (b) Yco₃²⁻ = 20 mol%.
- Fig. 3 Decomposition area of chloride calculated with Thermo-Calc¹⁵). Chloride phase solidified from molten salt, whose initial composition is the inside of this area, decomposes to KCl solid solution and NaCl solid solution.
- Fig. 4 Tie lines and liquidus lines at 923K with the eutectic line calculated with Thermo-Calc¹⁵).
- Fig. 5 Four composition areas of high strength expected by considering the deflection mechanism and some other factors which decrease strength.
- Fig. 6 Photographs of some defects on the cross section of cast specimens. (a) K⁺ composition in cation XK = 10 mol%, CO₃²⁻ composition in anion Yco₃²⁻ = 60 mol%. (b) XK = 60 mol%, Yco₃²⁻ = 20 mol%.
- Fig. 7 Bending strength map of Na⁺-K⁺-Cl⁻-CO₃²⁻ salt mixture with liquidus lines and eutectic line calculated with Thermo-Calc¹⁵).
- Fig. 8 Solidification structure of specimens with high strength. (a) K⁺ composition in cation XK = 10 mol%, CO₃²⁻ composition in anion Yco₃²⁻ = 60mol% (region A). (b) XK = 0 mol%, Yco₃²⁻ = 20mol% (region C).
- Fig. 9 SEM micrographs of broken surface (a)-(c), and results of EDX chemical analysis for selected area (d)-(f). (a) and (d): K⁺ composition in cation XK = 60 mol%, CO₃²⁻ composition in anion Yco₃²⁻ = 20 mol%. (b) and (e): XK = 80 mol%, Yco₃²⁻ = 20 mol%. (c) and (f): XK = 90 mol%, Yco₃²⁻ = 20 mol%.
- Fig. 10 K⁺ composition in cation for primary and eutectic chloride XK⁺(Chl.) calculated with Thermo-Calc¹⁵) plotted as a function of temperature. Initial cation compositions XK⁺ of salt mixture are (a) 90 mol%, (b) 80 mol%, (c) 70 mol% and (d) 60 mol%, and initial CO₃²⁻ composition in anion Yco₃²⁻ is 20 mol% for all.
- Fig. 11 Vickers hardenss of Na⁺-K⁺-Cl⁻-CO₃²⁻ salt mixture at the initial CO₃²⁻ composition Yco₃²⁻ is 20 mol%.
6. Conclusion and Discussion:
- Summary of Main Results: The study identified four composition areas within the KCl-NaCl-Na₂CO₃-K₂CO₃ system exhibiting high bending strength (up to >20 MPa). These areas are characterized by liquidus temperatures between 873K and 973K, and the coexistence of primary crystals and eutectic structures. Sodium content in the primary chloride phase was found to be a critical factor influencing strength.
- Academic Significance of the Research: This research provides valuable insights into the relationship between composition, thermodynamic properties, microstructure, and bending strength of multi-component salt systems for expendable cores. It demonstrates the effectiveness of combining thermodynamic calculations and experimental validation for material design in die casting applications. The study also highlights the importance of considering sodium content in chloride phases for strength optimization.
- Practical Implications: The identified high-strength composition areas with low liquidus temperatures offer promising candidates for developing water-soluble salt cores suitable for high-pressure die casting of undercut shapes. Specifically, the area "A" with strength above 20MPa and liquidus temperature between 873-973K is highly relevant for practical applications. These salt cores can potentially withstand the high injection speeds and pressures in die casting, enabling the production of complex aluminum parts.
- Limitations of the Research: The study focused primarily on bending strength. Other properties like thermal shock resistance, solubility, and core removal characteristics were not extensively investigated. The research also acknowledged that the strength in region D was only high in a limited portion of the two-phase separation region, requiring further investigation.
7. Future Follow-up Research:
- Directions for Follow-up Research:
- Further investigate the optimal sodium content in the primary chloride phase to maximize strength in high-potential composition areas.
- Explore the influence of cooling rate and solidification conditions on the microstructure and strength of salt cores in these systems.
- Evaluate other critical properties such as thermal shock resistance, solubility, and core removal efficiency for practical die casting applications.
- Investigate the detailed mechanism of strength enhancement in region D, particularly the role of sodium content and controlled two-phase separation.
- Areas Requiring Further Exploration:
- Optimizing the composition within the identified high-strength areas to achieve even higher bending strength and improved overall performance.
- Developing practical methods for manufacturing salt cores with these optimized compositions.
- Conducting die casting trials using salt cores made from these high-strength salt mixtures to validate their performance in industrial settings.
8. References:
- [List of references as provided in the original paper]
- 1) J. Yaokawa, D. Miura, K. Anzai, Y. Yamada and H. Yoshii J. JFS, to be published.
- 2) J. Yaokawa, K. Anzai, Y. Yamada, H. Yoshii and H. Fukui: J. JFS, 76 (2004) 823
- 3) J. Yaokawa, T. Sawada, K. Anzai, Y. Yamada, H. Yoshii and H. Fukui J. JFS, 78 (2006) 59
- 4) C. Hayashi, T. Yamazaki, T. Ishikuro and A. Urakami: ALUTOPIA, 35 (2006) 6, 22
- 5) N. Mantani and T. Touhata: SOKEIZAI, 36 (1995) 2, 14
- 6) R. Izawa, T. Takayama, Y. Mizukusa and T. Komazaki: Report of Japan Die Casting Association, JD02 (2002) 223
- 7) T. Manabe, M. Nitta and M. Yaguchi : SOKEIZ AI, 44 (2003) 12, 26
- 8) Yamazaki, A. Takai, O. Murakami, M. Kawabata, O. Ito and M. Kawabata: SAE Technical Paper 2004-01-1447
- 9) Y.Utsu, Japanese Patent Publication No.52-10803 (Mar. 26, 1977)
- 10) R.W.Foreman, U.S. Patent No.4, 840, 219 (Jun. 20, 1989)
- 11) General editors, L. P. Cook and H. F. McMurdie: Phase diagrams for ceramists vol.1, figure 1857 (Columbus, Ohio: American Ceramic Society) (1964)
- 12) General editors, L. P. Cook and H. F. McMurdie : Phase diagrams for ceramists vol. 7, figure 6976, 7058, 7060, 7256 (Columbus, Ohio: American Ceramic Society) (1989)
- 13) T. Sato: Technical Report of Tohoku Imp. Univ., XI (1934) 403
- 14) J. Yaokawa, K. Oikawa and K. Anzai: CALPHAD to be printed
- 15) B. Sundman, B. Jansson, J.-O. Andersson, CALPHAD 9 (1985) 153-190
- 16) Y. Kagawa and H. Hatta: Ceramic Matrix Composites-Tailoring Ceramic Composites, (Agune Shohusha) (1990) 124
- 17) S. Pehkonen J. Phys. D: Appl. Phys., 6 (1973) 544
9. Copyright:
This material is Yaokawa Jun, Miura Daisuke, Oikawa Katsunari, Anzai Koichi, Yamada Youji, and Yoshii Hiroshi's paper: Based on Bending Strength of Salt Core Comprised of KCl-NaCl-Na₂CO₃-K₂CO₃ Systems.
Paper Source:
This material was summarized based on the above paper, and unauthorized use for commercial purposes is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.