Beyond Trial-and-Error: Mastering AlSiCu Alloy Solidification with Dynamic Baseline Analysis
This technical summary is based on the academic paper "Fraction solid evolution characteristics of AlSiCu alloys - dynamic baseline approach" by P. Marchwica, J.H. Sokolowski, and W.T. Kierkus, published in the Journal of Achievements in Materials and Manufacturing Engineering (2011).
Keywords
- Primary Keyword: AlSiCu Alloy Solidification
- Secondary Keywords: Fraction Solid, Thermal Analysis, Dynamic Baseline, Casting Simulation, Aluminum Alloys, Solidification Modeling
Executive Summary
- The Challenge: Inaccurate casting simulations and suboptimal alloy design stemming from a poor understanding of the dynamic solidification process in complex AlSiCu alloys.
- The Method: Applying an improved thermal analysis technique, the "dynamic baseline approach," to precisely measure fraction solid evolution across a wide range of AlSiCu chemistries.
- The Key Breakthrough: The research established robust, quantifiable correlations between alloy chemistry (Si and Cu content) and key solidification events, enabling the creation of accurate predictive models.
- The Bottom Line: This methodology provides the precise, physically-grounded data needed to significantly enhance casting process simulations, accelerate alloy design, and reduce defects in high-performance AlSiCu components.
The Challenge: Why This Research Matters for HPDC Professionals
For decades, engineers have relied on simulations to predict the outcome of casting processes. However, the accuracy of these simulations is only as good as the input data. A critical parameter, fraction solid (fs) evolution—how much of the alloy is solid at a given temperature—has been notoriously difficult to determine accurately for complex industrial alloys like the AlSiCu family. Existing methods were often based on simplified assumptions or required complex, time-consuming experiments that were not representative of real-world conditions. This data gap leads to costly trial-and-error cycles, unexpected defects like porosity and hot tearing, and limitations in optimizing both the alloy chemistry and the casting process itself. This research was undertaken to develop a more precise and practical method for understanding and predicting the solidification behavior of these vital alloys.
The Approach: Unpacking the Methodology
The researchers developed and utilized a sophisticated methodology centered on the "dynamic baseline" concept, moving beyond the limitations of previous thermal analysis techniques. This approach provides a more accurate foundation for calculating the heat released during solidification and, consequently, the fraction solid evolution.
Method 1: Advanced Thermal Analysis Platforms
The study employed co-developed thermal analysis platforms, including the Universal Metallurgical Simulator and Analyzer (UMSA) and the Aluminum Thermal Analysis Platform (AlTAP). These systems were used to record high-resolution cooling curves from samples of twelve different hypoeutectic AlSiCu alloys with systematically varied Silicon (5-11 wt.%) and Copper (1-4 wt.%) content.
Method 2: The Dynamic Baseline (DBL) Calculation
The core of the methodology is the Newtonian Computer-Aided Cooling Curve Analysis, which calculates a "Dynamic Baseline." The DBL represents a hypothetical cooling curve of the material if no solidification occurred. By comparing the actual measured cooling curve to this calculated baseline, the researchers could precisely quantify the energy released at each stage of solidification. This method is powerful because the DBL is derived directly from the experimental data of the specific alloy and cooling conditions, eliminating the need for broad, often inaccurate assumptions.
The Breakthrough: Key Findings & Data
The comprehensive testing across a wide range of AlSiCu chemistries revealed clear, predictable trends in solidification behavior. This data provides a powerful tool for alloy designers and process engineers.
Finding 1: Alloy Chemistry Directly Governs Solidification Range
The study confirmed that alloy chemistry has a dramatic and predictable effect on key solidification temperatures. As shown across Figures 24-41, increasing the concentration of Silicon and Copper significantly lowered the liquidus temperature (the start of solidification) by as much as 64.9°C. Conversely, the solidus temperature (the end of solidification) remained relatively stable. This directly impacts the solidification range (Tliq - Tsol), which decreased from 142.1°C to 89.6°C with increasing solute content. A smaller solidification range is a critical factor in controlling defects like hot tearing.
Finding 2: Solidification Events are Highly Predictable
The research demonstrated that the relationships between alloy chemistry and metallurgical events are not random but follow strong, quantifiable trends. By subjecting the data to polynomial plane fitting, the researchers found excellent correlation coefficients (R²). As detailed in Table 4, 15 of the 18 measured characteristics (including temperatures and fraction solid values for key reactions) showed an R² value greater than 0.80 with a 2nd-degree plane fit. This high degree of correlation proves that the methodology can be used to create robust predictive models for fraction solid evolution based on an alloy's specific Si and Cu content.
Practical Implications for R&D and Operations
- For Process Engineers: This study's data and techniques can be used to dramatically improve the accuracy of casting process simulations. By inputting more precise fraction solid data, simulations can better predict solidification patterns, identify potential hot spots, and optimize parameters like cooling rates to minimize porosity and shrinkage.
- For Quality Control Teams: The data in Figure 36, which shows how the amount of AlSi eutectic formed (fAlSiG) varies with chemistry, can inform quality expectations. Understanding these relationships helps teams anticipate the final microstructure and associated mechanical properties for a given alloy composition.
- For Design Engineers: The findings provide a quantitative basis for designing and optimizing casting alloys. The relationships established between Si/Cu content and solidification events (e.g., solidification range, eutectic formation) allow designers to tailor alloy chemistry to achieve specific performance characteristics and improve castability.
Paper Details
Fraction solid evolution characteristics of AlSiCu alloys - dynamic baseline approach
1. Overview:
- Title: Fraction solid evolution characteristics of AlSiCu alloys - dynamic baseline approach
- Author: P. Marchwica, J.H. Sokolowski*, W.T. Kierkus
- Year of publication: 2011
- Journal/academic society of publication: Journal of Achievements in Materials and Manufacturing Engineering, VOLUME 47, ISSUE 2
- Keywords: Aluminum alloys; Baseline; Fraction solid; Thermal analysis
2. Abstract:
Purpose: The goal of the research presented in this paper is to gain a deeper understanding of dynamic solidification processes of metals and alloys through application of improved baseline and fraction solid methodologies to hypoeutectic aluminum-silicon alloys with varying concentrations of silicon and copper.
Design/methodology/approach: The paper makes use of numerical models developed at the University of Windsor, including Newtonian Computer-Aided Cooling Curve Analysis and the Silicon Equivalency algorithm. Co-developed thermal analysis platforms are also used, including the Universal Metallurgical Simulator and Analyzer (UMSA) and the Aluminum Thermal Analysis Platform (AlTAP).
Findings: This paper identifies key temperature and fraction solid values for hypoeutectic AlSiCu alloys across a wide range of chemistries. The paper also provides correlations whereby temperature/fraction solid values for metallurgical reactions can be predicted on the basis of chemistry.
Research limitations/implications: Future work for the project will expand upon the relationships between important metallurgical events and alloy chemistries and derive general trends to enhance predictive capabilities.
Practical implications: The data and techniques used in this paper may be used in order to improve simulations of casting processes. The relationships between solidification events and alloy chemistries will aid in the design and optimization of casting alloys and components.
Originality/value: This paper would be of value to members of the engineering community who need precise information about fraction solid for use in designing alloys or optimizing technology and simulations of casting processes.
3. Introduction:
Advanced Thermal Analysis of an alloy's cooling curve can provide vital quantitative metallurgical information, including the effects of chemical composition on solidification, fraction solid evolution, and dendrite coherency. To extract this data, it is necessary to compute a Dynamic Baseline curve (DBL), which represents the hypothetical cooling rate if no phase transformations occurred. Successful design of alloys and casting processes requires quantitative knowledge of fraction solid (fs) evolution, as it directly affects the as-cast structure and subsequent performance. This paper details the University of Windsor's methodology for fs analysis, which is utilized in its AlTAP and UMSA technology platforms, and provides examples for complex industrial alloys.
4. Summary of the study:
Background of the research topic:
The determination of fraction solid (fs) as a function of temperature is critical for modeling and optimizing casting processes. However, many existing methodologies for calculating fs from cooling curves rely on simplified assumptions or incomplete models. Specifically, early work in computer-aided cooling curve analysis often lacked a scientifically proven procedure for calculating the Dynamic Baseline (DBL), a necessary step for accurate fs determination.
Status of previous research:
Previous approaches to DBL calculation included Newtonian, Fourier, and empirical analyses, each with its own set of assumptions and limitations. Other methods for determining fs, such as DSC/DTA, quantitative metallography, and thermodynamic software packages (e.g., Scheil-Gulliver models), have restrictions related to sample size, equilibrium assumptions, or the complexity of multi-component industrial alloys. These methods often fail to capture dynamic events like undercooling, which are critical in real-world casting.
Purpose of the study:
The study aimed to gain a deeper understanding of the dynamic solidification processes in hypoeutectic AlSiCu alloys. This was achieved by applying an improved, Newtonian-based methodology for determining the Dynamic Baseline and fraction solid evolution across a wide range of alloy chemistries, thereby establishing correlations to enhance predictive capabilities for alloy design and process simulation.
Core study:
The core of the study involved the experimental analysis of twelve hypoeutectic AlSiCu alloys with nominal compositions of 5, 7, 9, and 11 wt.% Si combined with 1, 2, and 4 wt.% Cu. Using the AlTAP thermal analysis platform, cooling curves were recorded for each alloy. The dynamic baseline was calculated for each experiment, allowing for the subsequent determination of fraction solid evolution curves (fs vs. Temperature). Key metallurgical events (e.g., dendrite nucleation, eutectic reactions, solidus) were identified, and their characteristic temperatures and corresponding fraction solid values were cataloged. Finally, these data points were correlated with the alloy chemistries (Si and Cu content) to identify trends and establish predictive relationships.
5. Research Methodology
Research Design:
The research was designed as a systematic experimental investigation. A matrix of twelve AlSiCu alloys was created to cover a broad and relevant range of compositions within the 3XX series. The experimental conditions were carefully controlled to ensure repeatability and isolate the effects of chemistry on solidification. The study used a post-process analysis approach, where recorded cooling curve data was processed using a proprietary numerical model to calculate the DBL and fraction solid.
Data Collection and Analysis Methods:
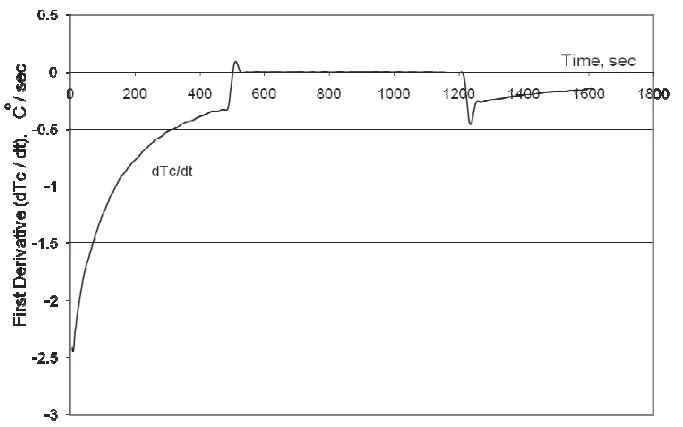
Data was collected using the Aluminum Thermal Analysis Platform (AlTAP). Test samples (approx. 300g) were melted and poured into thin-walled stainless steel cups, with temperature measured by a K-type thermocouple. The resulting temperature-time data was recorded at high speed. The primary analysis method was the Newtonian Computer-Aided Cooling Curve Analysis. This involved:
1. Recording the cooling curve, Tc(t).
2. Calculating its first derivative, dTc/dt.
3. Fitting a polynomial function to the single-phase (liquid and solid) portions of the dTc/dt curve to define the Dynamic Baseline (DBL) equation.
4. Calculating fraction solid by integrating the area between the experimental dTc/dt curve and the calculated DBL.
The resulting data for all alloys was then compiled and analyzed using Matlab to generate 3D surface plots and perform linear and polynomial plane fitting to determine correlation coefficients (R²).
Research Topics and Scope:
The research focused on hypoeutectic AlSiCu alloys. The scope included:
- Developing and validating the Dynamic Baseline (DBL) methodology for thermal analysis.
- Characterizing the fraction solid evolution for twelve distinct AlSiCu chemistries.
- Identifying key metallurgical reaction temperatures and their corresponding fraction solid values during solidification.
- Establishing correlations between alloy chemistry (specifically Si and Cu content) and the observed solidification characteristics.
The study did not investigate the influence of grain refiners or modifiers beyond the pre-modification of the initial ingots with strontium.
6. Key Results:
Key Results:
- The liquidus temperature decreases considerably (a range of 64.9°C) with an increase in SiEQ and Cu concentration, while the solidus temperature remains practically unchanged (range of 477.1-484.8°C). This leads to a reduction in the solidification range (Tliq - Tsol) from 142.1°C to 89.6°C.
- The nucleation temperature of the AlSi eutectic (TAlSiE NUC) shows a narrow range (22.3°C) and tends to decrease with an increase in Cu levels.
- The amount of AlSi eutectic formed (represented by fAlSiE G) strongly depends on alloy composition, decreasing from 66.2% (for 5 SiEQ / 1 Cu) to 17.7% (for 11 SiEQ / 4 Cu).
- Strong correlations were found between alloy chemistry and solidification events. Polynomial plane fitting of the data resulted in high correlation coefficients (R²), with 15 out of 18 parameters having an R² > 0.80, indicating high predictability.
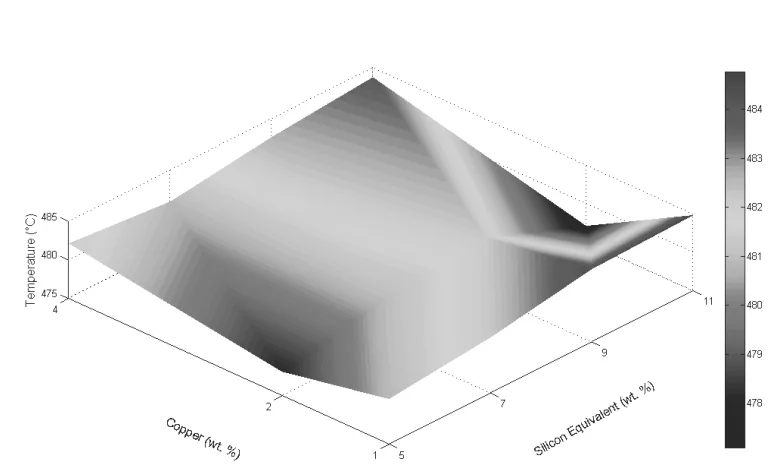
Figure Name List:
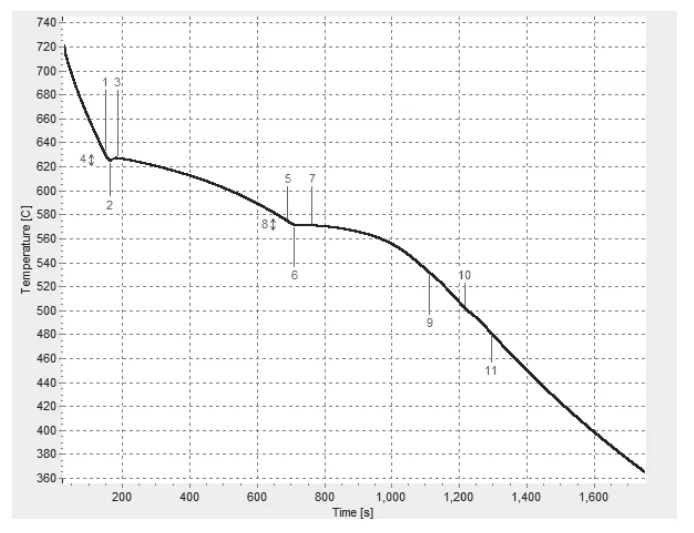
- Fig. 1. Temperature vs. Time Cooling Curve of a Nominal 5 wt.% Si and 1 wt.% Cu Aluminum Alloy
- Fig. 2. First Derivative vs. Temperature Cooling Curve of a Nominal 5 wt.% Si and 1 wt.% Cu Aluminum Alloy with Overlaid Dynamic Baseline
- Fig. 3. Fraction Solid Curve of a Nominal 5 wt.% Si and 1 wt.% Cu Aluminum Alloy with Metallurgical Events Indicated
- Fig. 4. Aluminum Silicon Binary Phase Diagram [48]
- Fig. 5. Cooling Curve for the 319.2 Aluminum Alloy
- Fig. 6. First Derivative of the Cooling Curve for the 319.2 Aluminum Alloy
- Fig. 7. Regression Equation of the Dynamic Baseline (DBL) for the 319.2 Aluminum Alloy
- Fig. 8. First Derivative of the Cooling Curve and the Dynamic Baseline (DBL) for the 319.2 Aluminum Alloy
- Fig. 9. Cooling Curve for Tin with a Purity of 99.95%
- Fig. 10. First derivative of the Cooling Curve for tin with a purity of 99.95%
- Fig. 11. Regression equation of the Dynamic Baseline (DBL) for tin with a purity of 99.95%
- Fig. 12. First derivative of the Cooling Curve and the dynamic Baseline (DBL) for pure tin
- Fig. 13. UMSA first derivatives of the heating and cooling curves for the 319.2 alloy test sample
- Fig. 14. Solidification of Near Pure Aluminum at Moderate Cooling Rates
- Fig. 15. Fraction Solid Curve for Industrially Pure Tin
- Fig. 16. Solidification of A356 Alloy at Medium Cooling Rates
- Fig. 17. Solidification of A356 Alloy at Moderate Cooling Rates
- Fig. 18. Solidification of A356 Alloy at Medium Cooling Rates
- Fig. 19. Solidification of A356 Alloy at Medium Cooling Rates
- Fig. 20. Matrix of Si and Cu Nominal Compositions (wt.%) used in experiments with overlaid compositions of selected 3XX alloys
- Fig. 21 - Fraction Solid Temperature Dependence for Nominal 1wt.% Cu and Nominal 5, 7, 9 and 11 wt.% Si
- Fig. 22. Fraction Solid Temperature Dependence for Nominal 2wt.% Cu and Nominal Si 5, 7, 9 and 11 wt.% Si
- Fig. 23. Fraction Solid Temperature Dependence for Nominal 4.wt.% Cu and Nominal 5, 7, 9 and 11 wt.% Si
- Fig. 24. Tα,DENNUC - Nucleation Temperature of αAl Dendrite Network (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 25. Tα,DENMIN - Temperature of αAl Dendrite Network Minimum (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 26. fsα,DENMIN - Fraction Solid at αAl Dendrite Network Minimum Temperature (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 27. Tα,DENG - Temperature of αAl Dendrite Network Growth (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 28. fsα,DENG - Fraction Solid at αAl Dendrite Network Growth Temperature (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 29. Tα,DENUNDER - αAl Dendrite Network Undercooling (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 30. fsα,DENUNDER - Fraction Solid Difference of αAl Dendrite Network Undercooling (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 31. TAlSiENUC - Temperature of AlSi Eutectic Nucleation (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 32. fsAlSiENUC - Fraction Solid at AlSi Eutectic Nucleation Temperature (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 33. TAlSiEMIN - Temperature of AlSi Eutectic Minimum (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 34. fsAlSiEMIN - Fraction Solid at AlSi Eutectic Minimum Temperature (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 35. TAlSiEG - Temperature of AlSi Eutectic Growth (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 36. fsAlSiEG - Fraction Solid at AlSi Eutectic Growth Temperature (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 37. TAlSiUNDER - AlSi Eutectic Undercooling (°C) vs. Cu (wt. %) and SiEQ (wt.%)
- Fig. 38. fsAlSiUNDER - Fraction Solid Difference of AlSi Eutectic Undercooling (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 39. TAlSiCuMg - Temperature of AlSiCuMg Eutectic (°C) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 40. fsAlSiCuMg - Fraction Solid at AlSiCuMg Eutectic Temperature (%) vs. Cu (wt.%) and SiEQ (wt.%)
- Fig. 41. Tsol - Solidus Temperature (°C) vs. Cu (wt.%) and SiEQ (wt.%)
7. Conclusion:
This paper demonstrates that it is possible and justifiable to use a single, unique function for the "overall heat transmission coefficient" U(Tc) to describe the energy loss from a solidifying test sample. This function allows for the derivation of a smooth, physically meaningful Dynamic Baseline (DBL) from experimental cooling curve data. This DBL enables the calculation of fraction solid based exclusively on the recorded temperature, applicable to any metal or alloy. The results from experiments on AlSiCu alloys showed that there are quantifiable, high-correlation trends between fraction solid evolution and alloy chemistry. This information is not only applicable to current commercial alloys but can also serve as a reasonable predictive model for fraction solid evolution of AlSi alloys beyond the specific chemistries investigated.
8. References:
- [1] M.B. Djurdjevic, W. Kasprzak, C.A. Kierkus, J.H. Sokolowski, Quantification of Cu enriched phases in synthetic 3XX aluminum alloys using the thermal analysis technique, AFS Transactions, 2001.
- [2] M. Djurdjevic, P. Gallo, H. Jiang, J.H. Sokolowski, Evaluation of strontium fading in the 319 aluminum alloy using thermal analysis, AFS Transactions 20 (2000) 485-486.
- [3] M. Djurdjevic, T. Stockwell and J. H. Sokolowski, The effect of strontium on the microstructure of aluminum-silicon and aluminum-copper eutectics in the 319 Alloy, International Journal of Cast Metals Research 12 (1999) 67-73.
- [4] M. Djurdjevic, W.T. Kierkus, R. Liliak, J.H. Sokolowski, Extended analysis of cooling curves, Proceedings of the 41st Conference of Metallurgists COM’2002, Montreal, 2002.
- [5] H. Jiang, W. Kierkus and J. H. Sokolowski, Dendrite coherency point determination using thermal analysis and rheological measurements, Proceedings of the International Conference on Thermophysical Properties of Materials TPPM’99, Singapore, 1999.
- [6] R. MacKay, M. Djurdjevic, J.H. Sokolowski, W. Evans, Using the method of a in-situ thermal analysis array in a cast section to assess riser feeding efficiency, Proceedings of the 131st TMS Annual Meeting, Seattle, Washington, 2002, 17-21.
- [7] R. MacKay, M. Djurdjevic, et al., Effect of cooling rate on fraction solid of metallurgical reactions in 319 alloy, AFS Transactions 25 (2000) 521-529.
- [8] X. Chen, W. Kasprzak, J.H. Sokolowski, Reduction of the heat treatment process for the Al-based alloys by utilization of heat from solidification process, Journal of Materials Processing Technology 176 (2006) 24-31.
- [9] L. Bäckerud, G. Chai, J. Tamminen, Solidification characteristics of aluminum alloys, AFS-Skanaluminium, 1990.
- [10] R. Mackay, J. Sokolowski, Experimental observations of dendrite coarsening and Al-Si eutectic growth in progressively quenched structures of Al-Si-Cu casting alloys, International Journal of Metalcasting Spring (2008) 57-80.
- [11] J. Barlow, D.M. Stefanescu, Computer aided cooling curve analysis revisited, unpublished, 1996.
- [And all other references from page 21 and 22.]
Expert Q&A: Your Top Questions Answered
Q1: Why is the "Dynamic Baseline" approach presented here considered superior to older thermal analysis methods?
A1: The Dynamic Baseline (DBL) approach is superior because it is derived directly from the experimental cooling data of the specific alloy under the specific test conditions. As explained in Section 5, it creates a physically meaningful, smooth curve representing the system's heat loss without phase change. Older methods often relied on arbitrary assumptions or linear approximations that do not accurately capture the complex, non-linear heat transfer dynamics, leading to errors in fraction solid calculation.
Q2: The study excludes Copper from the Silicon Equivalency (SiEQ) calculation. Why was this done?
A2: The paper states on page 9 that Copper was excluded from the SiEQ calculation for two main reasons. First, it allowed the researchers to present the fraction solid data as a clear function of the two primary alloying elements, Si and Cu, making the trends easier to visualize and analyze. Second, for the highly alloyed melts, including Cu could introduce complex interactions that were outside the intended scope of this particular investigation.
Q3: What is the practical significance of the high correlation coefficients (R²) found in the study?
A3: The high R² values, detailed in Table 4, are extremely significant. They demonstrate that the relationships between an alloy's chemistry (Si and Cu content) and its solidification behavior (key temperatures and fraction solid points) are strong and highly predictable. This validates the use of this data to build mathematical models that can accurately predict how a new or modified AlSiCu alloy will solidify, reducing the need for extensive physical trials in alloy development and simulation setup.
Q4: How reliable is the single-thermocouple method used in this research compared to methods using multiple thermocouples?
A4: The paper addresses this in Section 2.1. While some approaches use multiple thermocouples to map thermal gradients, the authors cite a recent study [21] suggesting that a single-thermocouple technique can be just as accurate, provided that the first and second derivatives of the cooling curve are utilized in the analysis. The methodology in this paper is based on this principle, ensuring reliable data from a less complex experimental setup.
Q5: How does this research help in predicting and controlling casting defects like hot tearing?
A5: This research provides critical data for controlling defects like hot tearing. Hot tearing is highly dependent on the solidification range (Tliq - Tsol) and the evolution of fraction solid in the final stages. The study quantifies exactly how Si and Cu content affect the solidification range (finding it decreases with more solutes) and the amount of eutectic liquid available late in solidification. This allows engineers to adjust alloy chemistry to achieve a more favorable solidification path that minimizes the window of vulnerability to tearing.
Conclusion: Paving the Way for Higher Quality and Productivity
The challenge of accurately predicting AlSiCu Alloy Solidification has long been a barrier to optimizing casting processes and materials. This research provides a significant breakthrough by establishing a robust, experimentally-grounded "dynamic baseline approach." The key finding—that solidification behavior can be accurately predicted from alloy chemistry—empowers engineers to move beyond costly trial-and-error and into an era of data-driven design. By leveraging these insights, R&D and operations teams can enhance simulation accuracy, accelerate the development of next-generation alloys, and ultimately reduce defects to improve component quality and manufacturing productivity.
"At CASTMAN, we are committed to applying the latest industry research to help our customers achieve higher productivity and quality. If the challenges discussed in this paper align with your operational goals, contact our engineering team to explore how these principles can be implemented in your components."
Copyright Information
This content is a summary and analysis based on the paper "Fraction solid evolution characteristics of AlSiCu alloys - dynamic baseline approach" by "P. Marchwica, J.H. Sokolowski, W.T. Kierkus".
Source: Journal of Achievements in Materials and Manufacturing Engineering 47/2 (2011) 115-136.
This material is for informational purposes only. Unauthorized commercial use is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.