Beyond the Surface: How Silicon Content Impacts Anodized HPDC Component Performance
This technical summary is based on the academic paper "INFLUENCE OF PRIMARY SILICON PRECIPITATES ON ANODIZED ALUMINUM ALLOYS SURFACE LAYER PROPERTIES" by Krzysztof LABISZ*, Jarosław KONIECZNY, Łukasz WIERZBICKI, Janusz ĆWIEK, and Anna BUTOR, published in TRANSPORT PROBLEMS (2018).
Keywords
- Primary Keyword: Anodizing Aluminum Castings
- Secondary Keywords: AlSi12 anodizing, AlSi8 anodizing, surface layer properties, wear resistance, silicon precipitates, HPDC finishing
Executive Summary
- The Challenge: Anodizing high-silicon aluminum die-cast alloys can result in non-uniform and less durable surface layers, compromising component performance in abrasive environments.
- The Method: The study compared the anodization of die-cast AlSi8 and AlSi12 alloys in a sulfuric acid electrolyte, analyzing the resulting microstructure, layer thickness, and wear resistance.
- The Key Breakthrough: The higher-silicon AlSi12 alloy produced a thinner but significantly more uniform and homogeneous anodic layer, which resulted in superior wear resistance compared to the AlSi8 alloy.
- The Bottom Line: For die-cast components requiring high wear resistance, selecting an alloy like AlSi12 can yield better performance after anodizing because the quality and homogeneity of the protective layer are more critical than its thickness.
The Challenge: Why This Research Matters for HPDC Professionals
Anodizing is a go-to process for enhancing the corrosion resistance, wear resistance, and aesthetic qualities of aluminum components. By electrochemically converting the surface into a hard aluminum oxide layer, we create a finish that is integral to the metal itself. This process is invaluable in transportation, construction, and countless other industries where durability and performance are paramount.
However, not all aluminum alloys anodize equally. The alloying elements that give materials like AlSi8 and AlSi12 their excellent casting properties—namely silicon—do not anodize. Instead, these silicon precipitates can disrupt the formation of the oxide layer, creating voids, inconsistencies, and a phenomenon known as "smutting," which affects both appearance and performance. For HPDC professionals, understanding how to manage the anodizing process for common high-silicon alloys is critical to ensuring the final product meets stringent quality and durability standards. This research tackles that exact problem, investigating how the silicon content directly influences the final properties of the anodized surface layer.
The Approach: Unpacking the Methodology
The researchers conducted a controlled experiment to isolate the effects of alloy composition on the anodized surface. Their methodology provides a clear and reliable basis for the findings.
Materials and Process:
- Alloys: The study focused on two common high-pressure die-cast alloys: AlSi8 (7.8% Si) and AlSi12 (12.5% Si).
- Anodizing Electrolyte: After preliminary tests showed that acids like oxalic, phosphoric, and chromic acid caused surface damage (Fig. 2), a 3% sulfuric acid (H₂SO₄) solution was chosen for its ability to produce a quality anodic layer.
- Anodizing Parameters: The process was tightly controlled, using a pulse current (2 A/dm² for 0.25 s and 1 A/dm² for 0.1 s) at a low temperature (-4 to 2 °C) to build the anodic layer. These specific parameters are detailed in Table 2 of the paper.
Analysis and Testing:
- Microstructure Analysis: The team used optical microscopy to examine the cross-section of the anodized layers, focusing on thickness, homogeneity, and the presence of pores or discontinuities.
- Wear Resistance Testing: An abrasive wear test was performed according to ISO 8251 standards. Samples were subjected to a 4.9 N load for 40 cycles/min, and performance was measured by the total mass loss in milligrams. This directly quantified the durability of the anodized surfaces.
The Breakthrough: Key Findings & Data
The investigation revealed a counter-intuitive but crucial relationship between silicon content, layer thickness, and wear resistance.
Finding 1: Higher Silicon Content Yields a Thinner but More Uniform Anodic Layer
The study found a stark difference in the physical characteristics of the anodized layers. While the lower-silicon AlSi8 alloy produced a significantly thicker coating, it was also far less consistent.
- As shown in Table 4, the anodized layer on AlSi8 had an average thickness of 32.3 µm, but with a large standard deviation of 9.74 µm, indicating high variability.
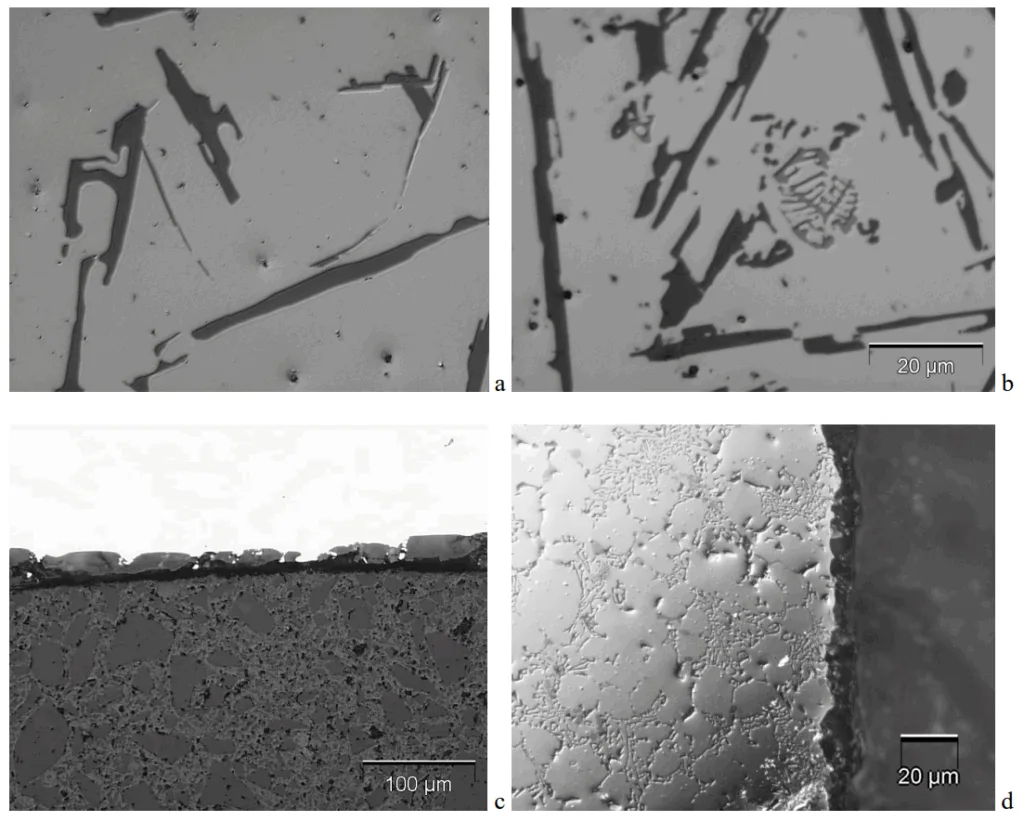
- In contrast, the anodized layer on the higher-silicon AlSi12 was much thinner at 9.7 µm, but it was remarkably more uniform, with a standard deviation of only 3.5 µm. The microstructure analysis (Figure 3d) confirmed the AlSi12 layer was more homogeneous with fewer, more regularly arranged pores.
Finding 2: Superior Homogeneity Leads to Better Wear Resistance
Despite its thinner protective layer, the anodized AlSi12 alloy significantly outperformed AlSi8 in durability tests. This finding underscores that layer quality is more important than sheer thickness.
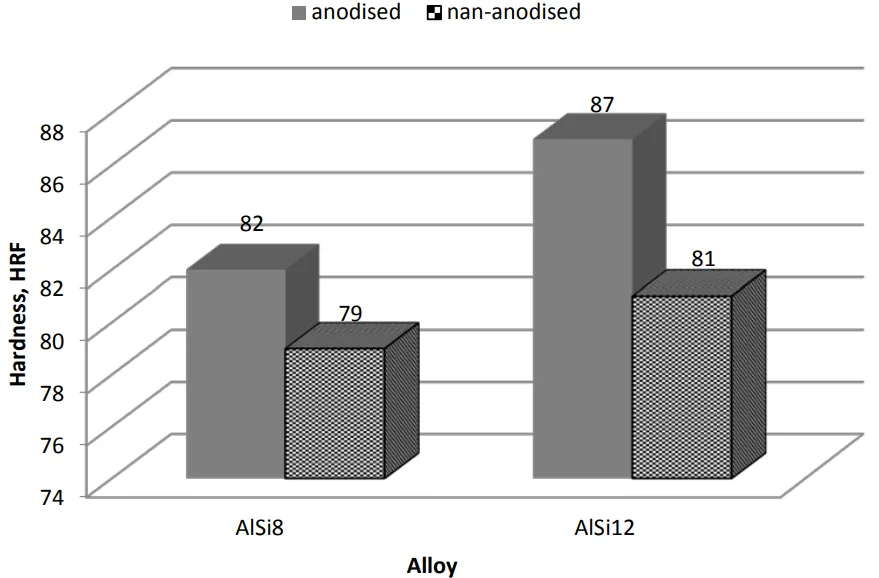
- According to the mass loss measurements in Table 5, the anodized AlSi8 sample lost 11.2 mg during the wear test.
- The anodized AlSi12 sample, with its thinner but more uniform layer, lost only 8.2 mg—a 27% improvement in wear resistance. Both anodized samples showed a dramatic improvement over their non-anodized counterparts, which lost 19.6 mg (AlSi8) and 16.7 mg (AlSi12), respectively.
Practical Implications for R&D and Operations
- For Process Engineers: This study suggests that when anodizing high-silicon alloys, aiming for maximum thickness may not yield the best performance. For AlSi12, focusing on process parameters that promote a uniform, dense, albeit thinner, layer is key to achieving superior wear resistance. The results also confirm that sulfuric acid is a robust electrolyte choice for these alloys.
- For Quality Control Teams: The data in Table 4 illustrates that standard deviation of the coating thickness is a critical quality metric. A low standard deviation, as seen with AlSi12, is a strong indicator of a homogeneous layer and, consequently, better mechanical performance. This could inform new quality inspection criteria beyond simple average thickness measurements.
- For Design Engineers: The findings indicate that for components intended for abrasive or high-wear environments, specifying AlSi12 over AlSi8 can provide a significant durability advantage after anodizing. This material choice, made early in the design phase, can lead to a more robust and reliable final product.
Paper Details
INFLUENCE OF PRIMARY SILICON PRECIPITATES ON ANODIZED ALUMINUM ALLOYS SURFACE LAYER PROPERTIES
1. Overview:
- Title: INFLUENCE OF PRIMARY SILICON PRECIPITATES ON ANODIZED ALUMINUM ALLOYS SURFACE LAYER PROPERTIES
- Author: Krzysztof LABISZ, Jarosław KONIECZNY, Łukasz WIERZBICKI, Janusz ĆWIEK, Anna BUTOR
- Year of publication: 2018
- Journal/academic society of publication: TRANSPORT PROBLEMS, Volume 13 Issue 2
- Keywords: anodization; aluminium alloys; microstructure; surface layer; wear resistance
2. Abstract:
In this work, we presented the influence of the anodizing method and parameters, as well as the chemical composition of the used aluminium alloys on the properties and microstructure of the anodic layer produced on aluminium alloys, in particular on the size and morphology of the primary silicon precipitates and the homogeneity of the resulting oxide coating. Aluminium alloys AlSi8 and AlSi12, produced using the die-casting method and subsequently subjected to anodic oxidation were used as test material. The microstructure of the obtained surface layer was analyzed by taking into account the primary silicon precipitates. The results of the hardness and abrasive wear test also show the influence of anodizing and electrolyte parameters on the structure and properties of the tested aluminium alloys.
3. Introduction:
Anodizing is an electrochemical process that converts an aluminum surface into aluminum oxide, increasing corrosion and wear resistance. It is widely used in many applications, including transport, due to its protective and aesthetic qualities. Unlike simple coatings, anodizing permanently alters the metal's outer structure, creating a much thicker and harder oxide layer than what forms naturally in air. The porous nature of this layer allows for dyeing. The purity of the aluminum alloy is critical, as alloying elements like silicon do not anodize and can create imperfections in the oxide film, affecting the final finish and its protective properties. This study investigates this effect on AlSi8 and AlSi12 alloys.
4. Summary of the study:
Background of the research topic:
Aluminum is a "strategic metal" widely used across industries, with transport being the single largest sector in Europe (nearly 40% of output). Anodized aluminum is favored for its lightweight properties, which increase fuel efficiency and reduce emissions. The process involves multiple steps: pretreatment, rinsing, etching, desmutting, anodizing, coloring, and sealing. Alloying elements like silicon can negatively impact the anodized finish, reducing corrosion and wear resistance. Cast aluminum parts are particularly challenging to anodize well due to their inherent porosity.
Status of previous research:
Previous research has established that anodizing increases the strength and corrosion resistance of aluminum. Experts predict aluminum content in cars will increase significantly. It is known that higher-purity alloys anodize better. Alloying elements like copper or silicon leave microscopic voids in the oxide film. The conversion of aluminum to aluminum oxide results in a volume increase, which is an important factor to consider. Antismutting agents are sometimes used during sealing to prevent the formation of boehmite, which can degrade surface quality.
Purpose of the study:
The study aims to investigate the influence of the anodizing method, its parameters, and the chemical composition of aluminum alloys (specifically AlSi8 and AlSi12) on the properties and microstructure of the resulting anodic layer. The focus is on how primary silicon precipitates affect the homogeneity of the oxide coating and its final hardness and wear resistance.
Core study:
The core of the study involved subjecting high-pressure die-cast AlSi8 and AlSi12 alloys to anodic oxidation. An optimal electrolyte (3% H₂SO₄) and specific process parameters were used. The resulting surface layers were then analyzed for their microstructure, thickness, and uniformity. Finally, abrasive wear tests were conducted to compare the performance of the anodized layers on both alloys against each other and against non-anodized control samples.
5. Research Methodology
Research Design:
The study employed a comparative experimental design. Two aluminum-silicon cast alloys with different silicon concentrations (AlSi8 and AlSi12) were selected. Both were subjected to the same anodizing process. The properties of the resulting surface layers were then measured and compared to determine the influence of the initial alloy composition.
Data Collection and Analysis Methods:
- Sample Preparation: Samples were die-cast, then prepared for analysis by cutting, mounting in resin, grinding, and polishing.
- Microstructural Analysis: An Olympus BX60M light microscope was used to obtain optical micrographs of the alloy microstructures and the cross-sections of the anodized layers. The "analySIS" program was used for image capture.
- Wear Testing: An ABR-8251 tester was used according to ISO 8251 standard. The wear resistance was quantified by measuring mass loss [mg] after the test.
- Anodizing Process: Anodizing was performed in a 3% H₂SO₄ electrolyte at -4 to 2 °C with a specific pulse current. Other electrolytes (H₂C₂O₄, H₃PO₄, CrO₃) were initially tested but discarded due to poor results.
Research Topics and Scope:
The research focused on high-pressure die-cast AlSi8 and AlSi12 alloys. The investigation covered the influence of the alloy's chemical composition (specifically silicon content) on the resulting anodized layer's microstructure (homogeneity, thickness) and mechanical properties (wear resistance). The scope was limited to a specific set of anodizing parameters using a sulfuric acid electrolyte.
6. Key Results:
Key Results:
- A significant color change (graying) was observed on the surfaces after anodizing, attributed to "silicon smutting," which was more pronounced on the higher-silicon AlSi12 alloy.
- The anodized layer on the AlSi12 alloy was thinner (9.7 µm) but significantly more homogeneous and uniform compared to the layer on the AlSi8 alloy, which was thicker (32.3 µm) but less uniform.
- Despite having a thinner layer, the anodized AlSi12 alloy exhibited higher wear resistance (8.2 mg mass loss) than the anodized AlSi8 alloy (11.2 mg mass loss).
- Anodizing significantly improved wear resistance for both alloys compared to their non-anodized states. Anodized AlSi8 showed a 43% reduction in mass loss, while anodized AlSi12 showed a 51% reduction.
Figure Name List:
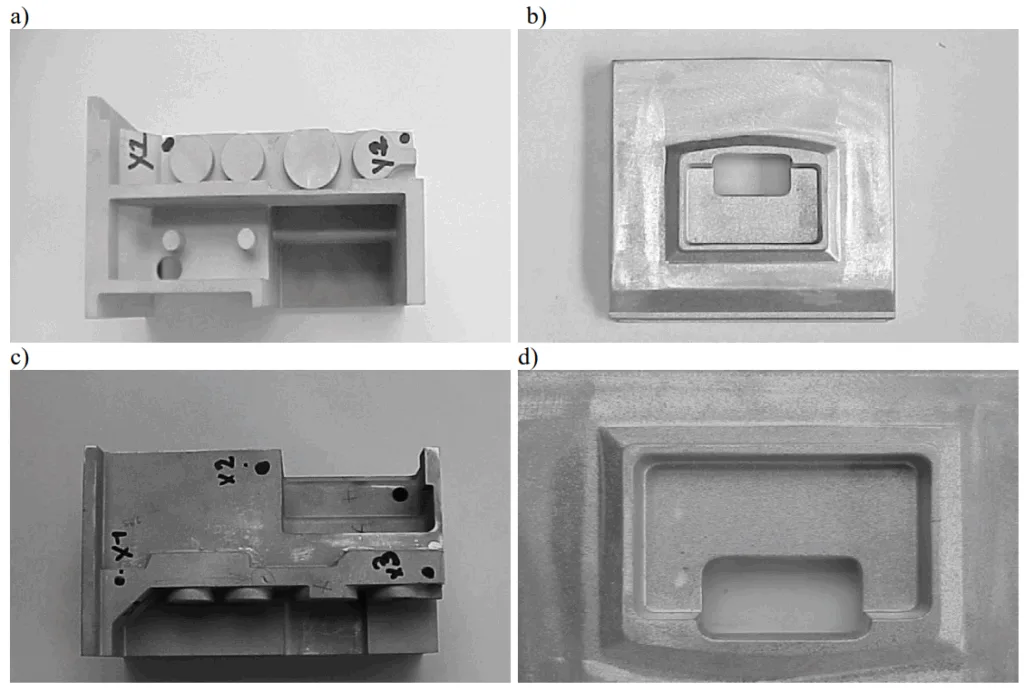
- Fig. 1. Parts of housing used for anodizing in the state before anodizing: a) AlSi12, b) AlSi8 and after anodizing: c) AlSi12, d) AlSi8
- Fig. 2. Element damage occurred after anodizing in other acids than H₂SO₄
- Fig. 3. Microstructure of the a) AlSi8 and b) AlSi12 cast aluminium alloy used for anodizing. Cross-section of the obtained surface layer after anodizing: c) AlSi8 and d) AlSi12
- Fig. 4. Mass loss measured during the wear test of the anodized and non-anodized AlSi8 and AlSi12 alloys
7. Conclusion:
The investigated AlSi8 and AlSi12 cast aluminum alloys are both suitable for anodic oxidation. However, the alloy composition significantly impacts the outcome. The AlSi12 alloy produces a thinner (9.7 µm) but more homogeneous and uniform alumina layer compared to the AlSi8 alloy (32.2 µm). The wear investigation results show that this superior layer structure on AlSi12 leads to better abrasion resistance. A large degree of silicon smutting was observed, causing a gray to black surface color. The authors recommend that an anodic desmutting process should be applied after anodizing to remove silicon or carbon smut formed during the acid treatment.
8. References:
- Tichelaar, L.E. & Thompson, F.D. & Terryn, G.E. & at al. A transmission electron microscopy study of hard anodic oxide layers on AlSi(Cu) alloys. Electrochimica Acta. 2004. Vol. 49. P. 3169-3177.
- Vrublevsky, I. & Parkoun, V. & Schreckenbach, J. at al. Effect of the current density on the volume expansion of the deposited thin films of aluminium during porous oxide formation. Applied Surface Science. 2003. Vol. 220. 51-59.
- Vrublevsky, I. & Parkoun V. & Sokol, V. The study of the volume expansion of aluminium during porous oxide formation at galvanostatic regime. Applied Surface Science. 2004. Vol. 222. P. 215-225.
- Gwoździk, M. & Nitkiewicz, Z. Wear resistance of steel designed for surgical instruments after heat and surface treatments. Archives of Metallurgy and Materials. 2009. Vol. 54. No. 1. P. 241-246.
- Włodarczyk-Fligier, A. & Dobrzański, L.A. & Konieczny, J Ceramic particles. Journal of Achievements in Materials and Manufacturing Engineering. 2012. Vol. 51. No. 1. P. 22-29.
- Konieczny, J. & Dobrzański, L.A. & Labisz, K. & at al. The influence of cast method and anodizing parameters on structure and layer thickness of aluminium alloys. Journal of Materials Processing Technology. 2004. Vol. 157-158. P. 718-723.
- Labisz, K., & Tański, T. & Janicki, D. HPDL energy absorption on anodised cast Al-Si-Cu alloys surfaces during remelting, Archives of Foundry Engineering. 2012. Vol. 12. No. 2. P. 45-48.
- Juchim, S. Nanoporous structure of alumina in one- and two-step anodisation process. Przegląd Elektrotechniczny. 2013. Vol. 89. No. 7. P. 155-157.
- Posmyk, A. & Bogdan-Włodek, A. Thermal composite coatings improving quality of technical means of transport. Scientific Journal of Silesian University of Technology. Series Transport. 2015. Vol. 87. P. 21-26.
- Gilbert Kaufman, J. Properties of aluminum alloys: Fatigue Data and the Effects of Temperature, Product Form, and Processing. ASM International. 2008.
- Davis, J.R. Aluminum and Aluminum Alloys, ASM International, 1993.
- McQueen, J.H. & Spigarelli, S. & Kassner, M.E. & Evangelista, E. Hot Deformation and Processing of Aluminum Alloys. CRC Press Taylor & Francis Group. 2011.
- Totten, G.E. & MacKenzie D.S. Handbook of Aluminum: Volume 2: Alloy Production and Materials Manufacturing. Marcel Dekker Inc. 2005.
- Scully, J.R. & Silverman, D.C. & Kendig, M.W. Electrochemical Impedance: Analysis and Interpretantion. ASTM. 1993.
- Henley, V.F. Anodic Oxidation of Aluminium and Its Alloys. Pergamon Press. 2000.
- Brace, A.W. The technology of anodizing aluminium. Aluminum Anodizers. 2000.
- Sheasby, P.G. & Pinner, R. The Surface Treatment and Finishing of Aluminum and Its Alloys. Tom 2. ASM International. 2001.
- Kawai, S. Anodizing and coloring of aluminum alloys. Finishing Publications. 2002.
- Ghali, E. Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance and Testing. Jon Wiley & Sons, INC. 2010.
- Skoneczny, W. Shaping the properties of aluminum and its alloys by hard anodizing. Wydawnictwo Politechniki Łódzkiej. Filia w Bielsku-Białej. 2001.
- Polski Komitet Normalizacyjny. Aluminum and aluminum alloys - anodic oxidation - p. 1: Methods for characterizing decorative and protective anodic oxide coatings on aluminum PN-EN 12373-1. PKN, 2004.
- Takadoum, J. Nanomaterials and Surface Engineering. ISTE Ltd. and John Wiley and Sons, Inc. 2010.
- Takadoum, J. Materials and Surface Engineering in Tribology. ISTE Ltd. and John Wiley and Sons. Inc, 2013.
- Tiwari, A. & Wang, R. & Wie, B. Advanced Surface Engineering Materials. Scrivener Publishing LCC. 2016.
- Grandfield, J. Light Metals 2014. Springer International Publishers. 2016.
- Minet, A. The Production of Aluminum and Its Industrial Use. Fb & c Limited. 2016.
- Cabot, T. & Tetrault, J. & Dong-Jin, S. Microcrystalline anodic coatings and related methods therefor. Sanford Process Corp. 2010.
- Lumley, R. Fundamentals of Aluminium Metallurgy: Production, Processing and Applications. Woodhead Publishing Limited. 2010.
- Dudin, M.N. & Voykova, N.A. & Frolova, E.E. & Artemieva, J.A. & Ruskova, E.P. & Abashidze, A.H. Modern trends and challenges of development of global aluminum. MEТАВК. 2017. Vol. 56(1-2). Р. 255-258.
- Kodres, C.A. & Polly, D.R. & Hoffard, T.A. & Anguiano, G.D. Surface Quality Impact of Replacing Vapor Degreasers with Aqueous Immersion Systems. Technical Report TR-2067-ENV Naval Facilities Engineering Service Center. 1997.
Expert Q&A: Your Top Questions Answered
Q1: Why was sulfuric acid chosen as the electrolyte over other acids like oxalic or phosphoric acid?
A1: The paper states that initial tests were conducted with several electrolytes, including 3% H₂C₂O₄, 4% H₃PO₄, and 3% CrO₃. However, these other acids resulted in "damages or discontinuities of the obtained alumina surface," as shown in Figure 2. Sulfuric acid (3% H₂SO₄) was ultimately chosen for the final investigation due to the superior "initial quality of the obtained anodic layer."
Q2: The paper mentions "silicon smutting." What is this phenomenon and what was its effect on the samples?
A2: Silicon smutting is identified as the primary cause of the characteristic gray color observed on the surfaces after anodizing. It is caused by the silicon atoms present in the alloy that do not anodize and become part of the created alumina layer. The effect was more intense on the AlSi12 alloy, which appeared darker (Fig. 1c) than the AlSi8 alloy (Fig. 1d) due to its higher silicon content.
Q3: Is a thicker anodized layer always better for wear resistance?
A3: No, this study demonstrates that is not the case. The AlSi8 alloy produced a much thicker layer (32.3 µm) but exhibited worse wear resistance, with 11.2 mg of mass loss. In contrast, the AlSi12 alloy had a significantly thinner layer (9.7 µm) but showed superior wear resistance, with only 8.2 mg of mass loss. This indicates that the homogeneity and uniformity of the layer are more critical for durability than its absolute thickness.
Q4: What was the key difference in the microstructure of the anodized layers between AlSi8 and AlSi12?
A4: The metallographic observations showed a clear difference in layer quality. The cross-section of the anodized AlSi12 layer (Fig. 3d) exhibited "much higher homogeneity," characterized by a low amount of small pores with a more regular arrangement. The layer on AlSi8 (Fig. 3c), while thicker, was less uniform, as confirmed by its much higher standard deviation in thickness measurements (Table 4).
Q5: The conclusion recommends "anodic desmutting." What is its purpose in the process?
A5: The authors recommend applying an anodic desmutting step after the main anodizing process. Its purpose is "to remove any silicon or carbon smut formed during acid treatment." This is a direct response to the "relatively large smutting" observed on the treated parts, which caused the gray and black surface coloration. This step would likely be for improving the cosmetic finish and surface cleanliness.
Conclusion: Paving the Way for Higher Quality and Productivity
This research provides a critical insight for anyone involved in Anodizing Aluminum Castings: for high-silicon alloys, the quality of the protective layer trumps its quantity. The study clearly demonstrates that the higher silicon content in AlSi12, while producing a thinner anodic layer, results in a more uniform and homogeneous surface that delivers superior wear resistance. This challenges the conventional wisdom that a thicker coating is always better and highlights the importance of alloy selection and process control in achieving desired performance outcomes.
At CASTMAN, we are committed to applying the latest industry research to help our customers achieve higher productivity and quality. If the challenges discussed in this paper align with your operational goals, contact our engineering team to explore how these principles can be implemented in your components.
Copyright Information
This content is a summary and analysis based on the paper "INFLUENCE OF PRIMARY SILICON PRECIPITATES ON ANODIZED ALUMINUM ALLOYS SURFACE LAYER PROPERTIES" by "Krzysztof LABISZ, Jarosław KONIECZNY, Łukasz WIERZBICKI, Janusz ĆWIEK, Anna BUTOR".
Source: https://doi.org/10.20858/tp.2018.13.2.11
This material is for informational purposes only. Unauthorized commercial use is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.