Jun Yaokawa, Daisuke Miura, Koichi Anzai, Youji Yamada, Hiroshi Yoshii
Abstract
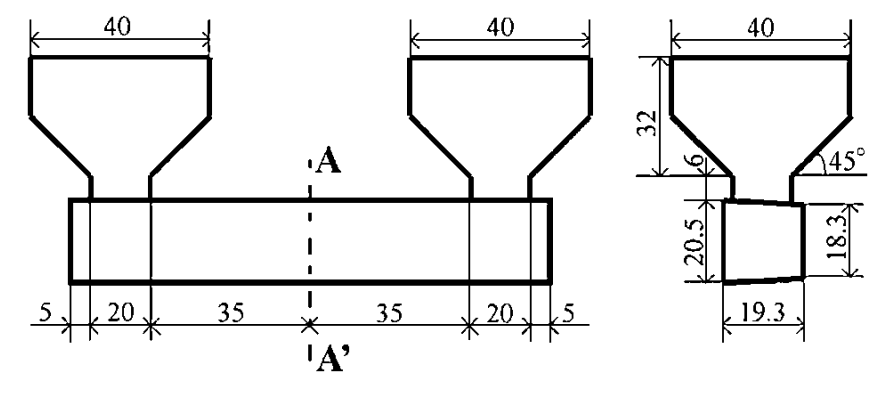
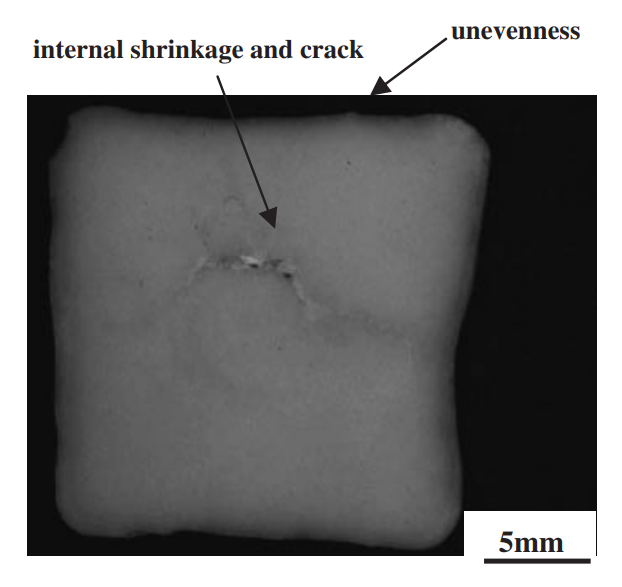
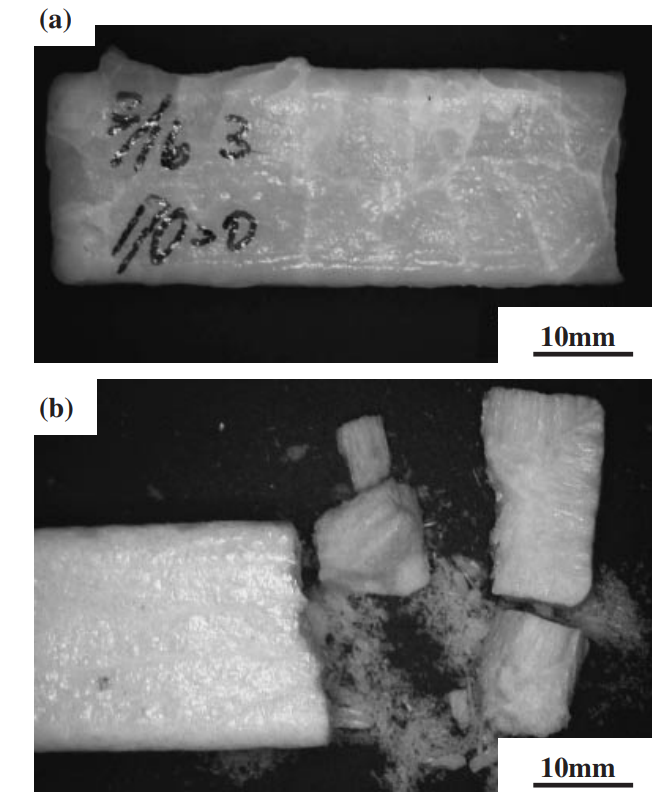
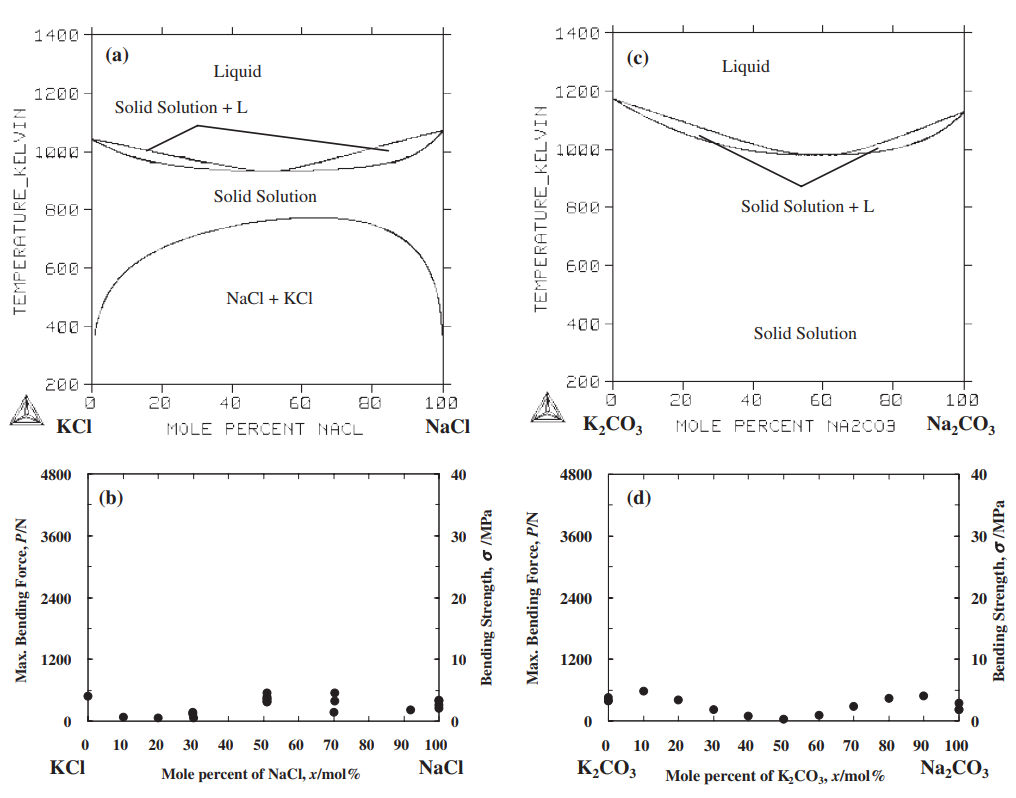
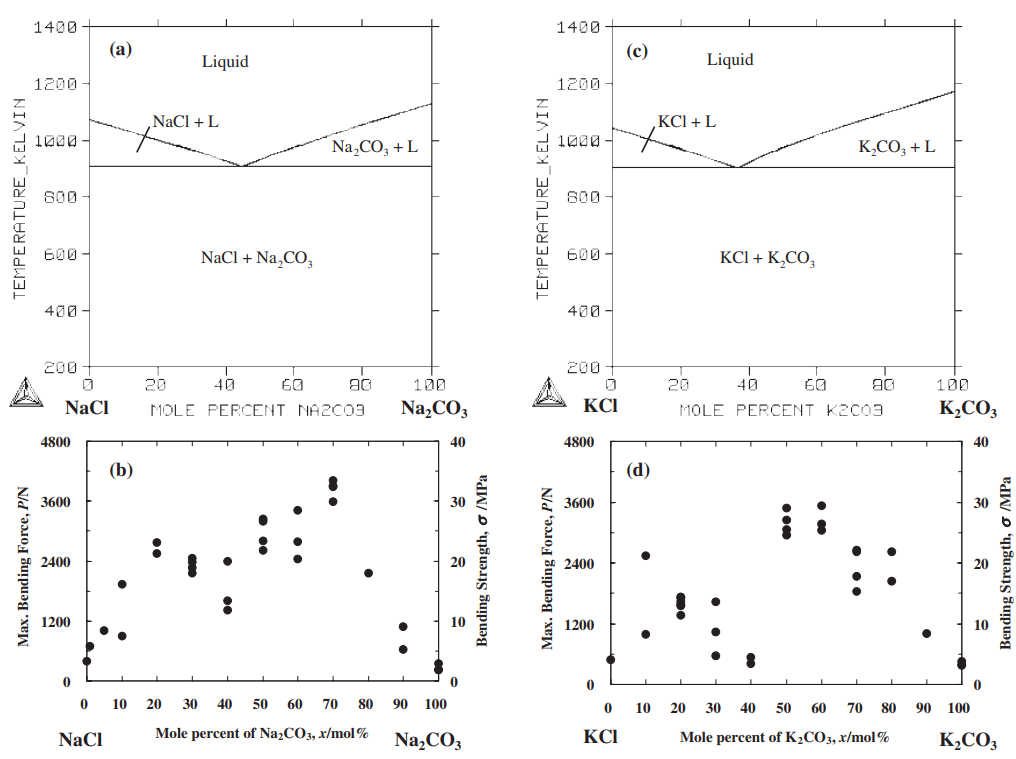
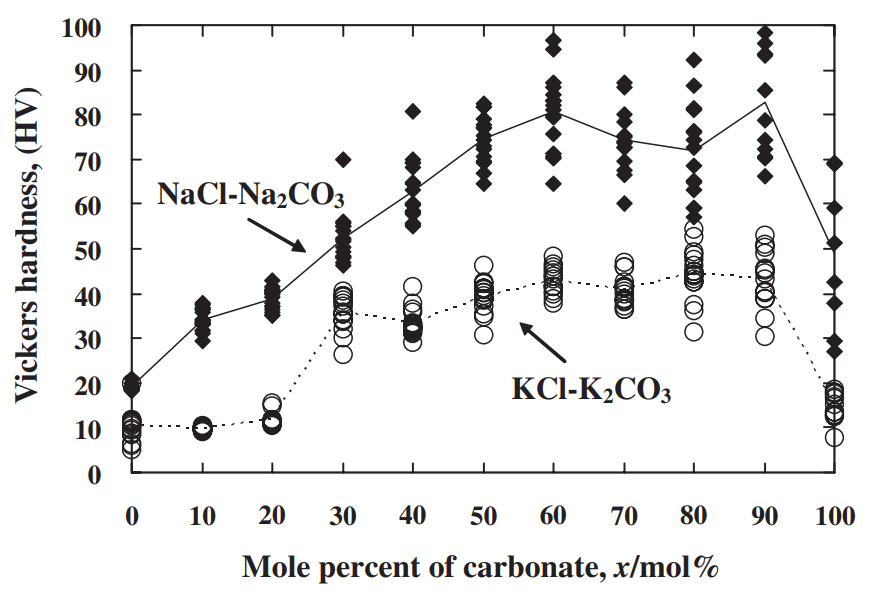
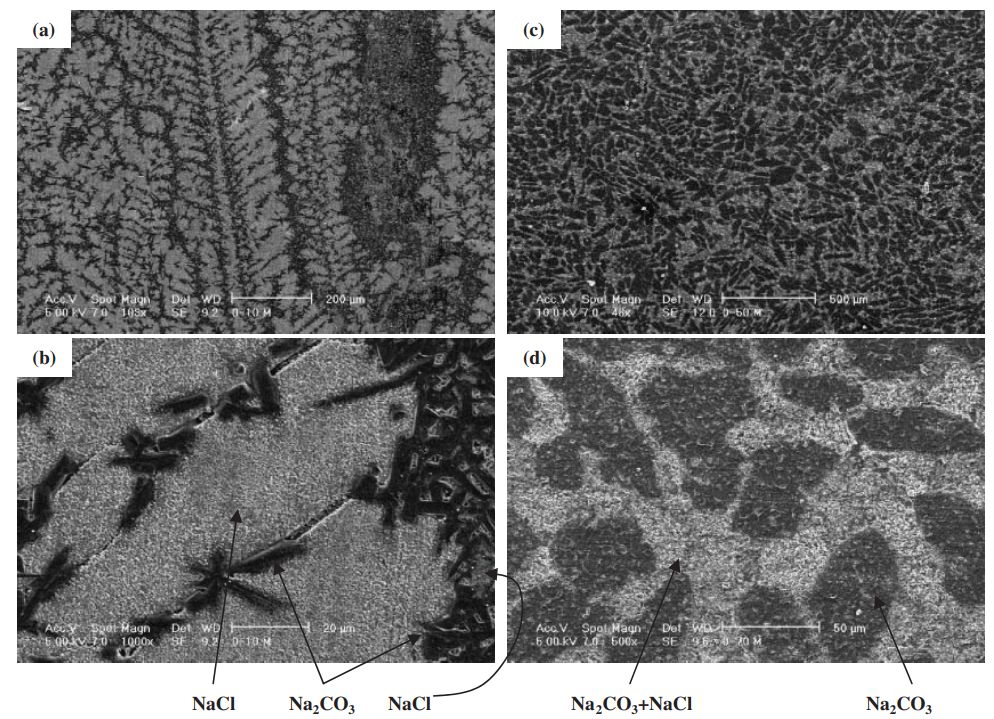
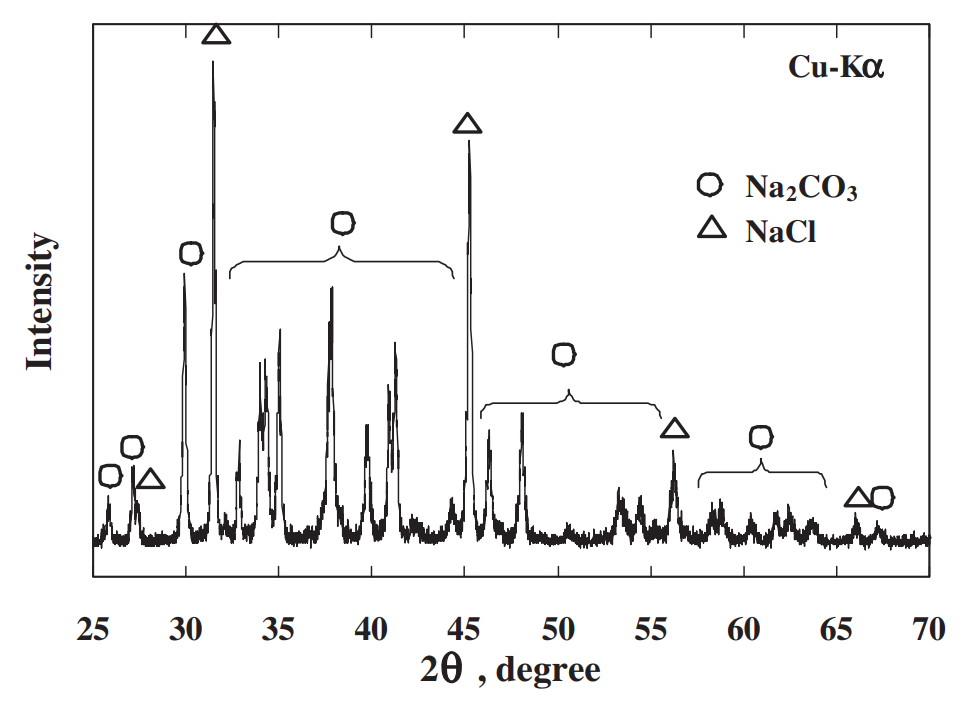
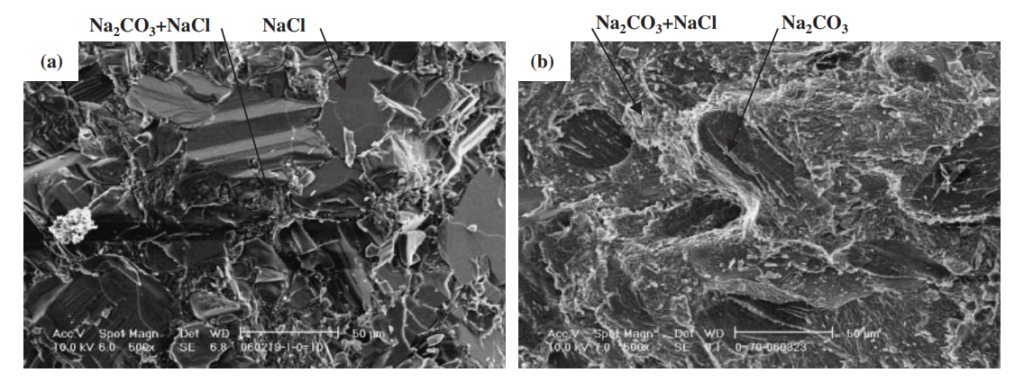
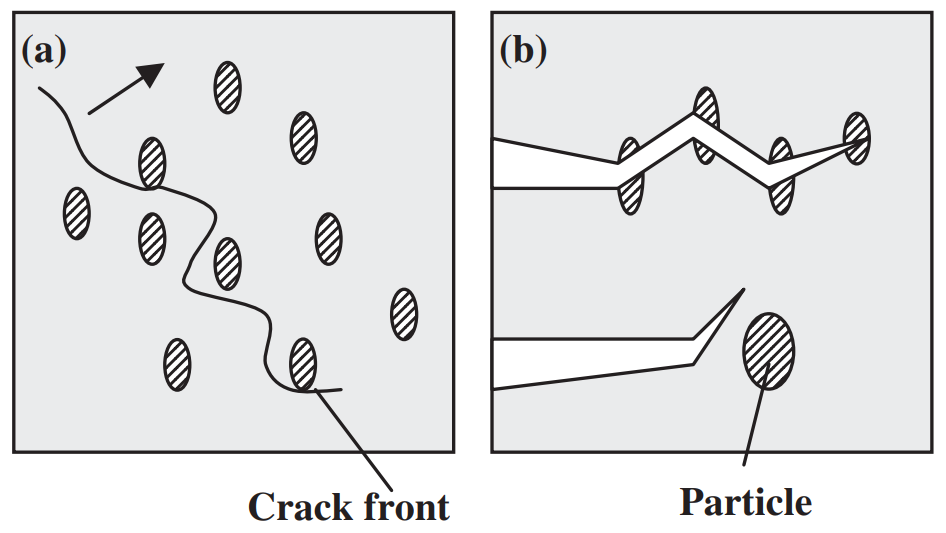
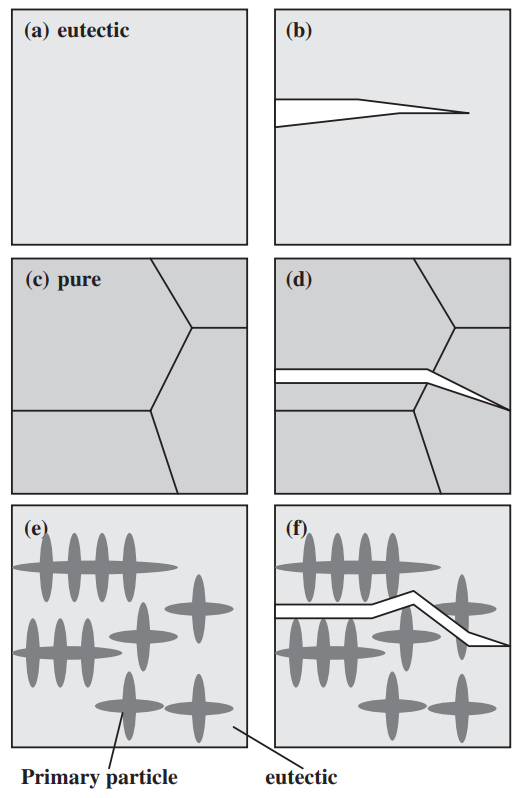
The strength of four binary systems NaCl–Na2CO3, KCl–K2CO3, KCl–NaCl and K2CO3–Na2CO3 was investigated in order to develop expendable salt core for high pressure die casting processes. Four point bending test was conducted to determine the strength of specimens made from molten salts by using the permanent mold casting technique. The strength of the system NaCl–Na2CO3 was over 20 MPa at the Na2CO3 composition between 20 mol% and 30 mol%, and between 50 mol% and 70 mol%. The highest strength was about 30 MPa at the composition of NaCl–70 mol%Na2CO3. This strength was 5 times as high as that of commonly used sand cores. The system KCl–K2CO3 also showed 20 MPa in strength. It was observed that there were the primary particles surrounded by the eutectic structure in the solidification structure of the systems NaCl–Na2CO3 and KCl–K2CO3 at the composition where the peak strength was obtained. The presence of the primary particles played an important role to strengthen the structure because the primary particles can prevent or deflect the crack propagation. In contrast to these binary systems, the systems KCl–NaCl and K2CO3–Na2CO3 were very brittle due to the phase decomposition or other solid–solid phase transformation of the solid solution phase. The strength of these systems was under 6 MPa.
일반적으로 사용되는 모래 코어만큼 높습니다. 시스템 KCl–K2CO3도 20MPa의 강도를 보였습니다. NaCl-Na2CO3계와 KCl-K2CO3계의 응고구조는 피크강도가 얻어지는 조성에서 공융구조로 둘러싸인 1차 입자가 존재함을 관찰하였다. 1차 입자의 존재는 1차 입자가 균열 전파를 방지하거나 편향시킬 수 있기 때문에 구조 강화에 중요한 역할을 했습니다. 이러한 이원 시스템과 대조적으로 시스템 KCl–NaCl 및 K2CO3–Na2CO3는 고용상의 상 분해 또는 기타 고체-고체 상 변환으로 인해 매우 부서지기 쉽습니다. 이 시스템의 강도는 6 MPa 미만이었습니다.
Keywords
salt core, expendable core, carbonate, chloride, die casting, strength, microstructure
REFERENCES
1) T. Manabe, M. Nitta and M. Yaguchi: SOKEIZAI 44 (2003) 26–30.
2) Yamazaki, A. Takai, O. Murakami, M. Kawabata, O. Ito and M.
Kawabata: SAE Technical Paper 2004-01-1447.
3) Y. Mizukusa: U.S. Patent No. 5,690,159 (Jun. 20, 1997).
4) K. Fujita and K. Atake: Japanese Patent Publication No. H9-108779
(Apr. 28, 1997).
5) T. Sakoda: U.S. Patent No. 3,963,818 (Jun. 15, 1976).
6) W. A. Gibbons: U.S. Patent 1,523,519 (Feb. 12, 1924).
7) F. D. Obolentsev, V. A. Voznesenskii, L. A. Ivanova and I. D. Chmykh:
Chem. Petroleum Eng. 13 (1977) 519–521.
8) Y. Utsu: Japanese Patent Publication No. 52–10803 (Mar. 26, 1977).
9) R. W. Foreman: U. S. Patent No. 4,840,219 (Jun. 20, 1989).
10) A. D. Ackerman, H. A. Aula: U.S. Patent 4,446,906 (May 8, 1984).
11) Y. Urabe and K. Takeda: Japanese Patent Publication, No. 48-39696
(Nov. 26, 1973).
12) J. Yaokawa, K. Anzai, Y. Yamada, H. Yoshii and H. Fukui: J. JFS, 76
(2004) 823–829.
13) J. Yaokawa, K. Anzai, Y. Yamada and H. Yoshii: NADCA Transactions T05-083 (2005).
14) General editors, L. P. Cook and H. F. McMurdie: Phase diagrams for
ceramists vol. 1, Fig. 1857 (The American Ceramic Society, Columbus,
Ohio, 1964).
15) B. Sundman, B. Jansson, J.-O. Andersson: Calphad 9 (1985) 153–190.
16) S. Dong, E. Niyama and K. Anzai: Cast Metals 6 (1993) 115–120.
17) S. Pehkonen: J. Phys. D: Appl. Phys. 6 (1973) 544–551.
18) L. P. Cook and H. F. Mcmurdie: Phase Diagrams for Ceramists vol. 7,
Fig. 6976 (The American Ceramic Society, Columbus, Ohio, 1976)
pp. 16–17.
19) A. Reisman: J. Am. Chem. Soc. 81 (1959) 807–811.
20) Y. Kagawa and H. Hatta: Ceramic Matrix Composites–Tailoring
Ceramic Composites, (Agune Shohusha,1990) 120–157.
21) C. Gandhi and M. F. Ashby: Acta Metall. 27 (1979) 1565.
22) E. Scheil: Z. Metallk. 34 (1942) 70.
23) J. Sangster and A. D. Pelton: J. Phys. Chem. Ref. Data 16 (1987) 509.