Unlocking Flawless Finishes: A Deep Dive into the Science of Electroplating on Zinc Die Casting
This technical summary is based on the academic paper "Special Aspects of Electrodeposition on Zinc Die Castings" by Valeriia Reveko and Per Møller, published in NASF SURFACE TECHNOLOGY WHITE PAPERS (2018). It has been analyzed and summarized for technical experts by CASTMAN.
Keywords
- Primary Keyword: Zinc Die Casting Surface Finish
- Secondary Keywords: electroplating on zinc, die casting defects, Zamak 5 properties, surface preparation for plating, die cast skin, porosity in die casting
Executive Summary
- The Challenge: Unpredictable surface variations, hidden porosity, and process-related contaminants in zinc die castings frequently lead to costly and unreliable electroplating defects.
- The Method: Researchers used Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS) to analyze the surface morphology and chemical composition of Zamak 5 die castings before and after corrosion testing and mechanical grinding.
- The Key Breakthrough: The study quantifies how the die casting process creates a protective but delicate "die cast skin" over a porous core, and reveals how surface contaminants like aluminum oxide and release agents compromise plating adhesion.
- The Bottom Line: A successful Zinc Die Casting Surface Finish hinges on carefully controlled post-casting treatments, specifically grinding, that meticulously clean the surface without breaching the critical "die cast skin."
The Challenge: Why This Research Matters for HPDC Professionals
Zinc die casting is a popular choice for manufacturing consumer goods, automotive parts, and other components due to its excellent castability, dimensional stability, and moderate solidification temperature, which translates to energy and cost savings. However, when these components require a high-quality protective and decorative electroplated finish, manufacturers often face hidden challenges.
The primary issue is that unpredictable variations in surface composition and morphology, caused by the die casting process itself, can severely affect the quality and performance of the plating. This research was necessary to overcome these issues by conducting a thorough analysis of the zinc die cast surface, linking its properties directly to the casting process and identifying the critical factors that lead to plating failures. For any engineer dealing with blistering, delamination, or poor adhesion on plated zinc parts, this paper addresses the root cause of the problem.
The Approach: Unpacking the Methodology
To investigate the challenges of plating on zinc, the researchers focused on Zamak 5 as a representative alloy for this class of materials. The methodology involved a multi-stage analysis:
- Corrosion Susceptibility Test: Samples were immersed in a 0.5M sodium chloride solution for up to 60 minutes. The surface was analyzed before and after immersion to demonstrate the material's inherent tendency to corrode.
- Surface and Compositional Analysis: The researchers used Scanning Electron Microscopy (SEM) to visualize the surface morphology and identify corrosion damage. Energy Dispersive X-ray Spectroscopy (EDS) was employed to quantify the elemental composition of the surface, revealing changes caused by corrosion and identifying contaminants.
- Microstructural Examination: Cross-sections of die cast samples were analyzed to reveal the internal structure, specifically the difference between the dense outer "die cast skin" and the porous inner bulk material.
- Post-Treatment Evaluation: The study examined the effect of grinding, a common post-treatment operation, on the surface composition to determine its effectiveness as a pretreatment step for electroplating.
This systematic approach allowed the researchers to isolate and identify the distinct factors—from alloy composition to process-induced defects—that impact the final Zinc Die Casting Surface Finish.
The Breakthrough: Key Findings & Data
The research yielded several critical findings that directly impact how zinc die cast parts should be handled and prepared for electroplating.
Finding 1: Inherent Corrosion Risk from Alloying Elements
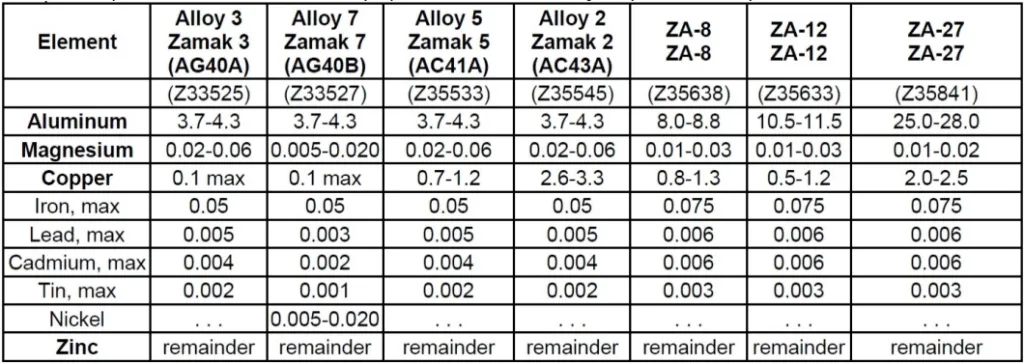
The study confirmed that the very elements that give Zamak alloys their desirable properties can also promote corrosion. EDS analysis of a sample surface before and after immersion in a salt solution showed a clear example of selective corrosion.
As shown in Table 2, after 60 minutes of exposure, the relative weight percentage of zinc decreased from 95.1% to 94.6%, while the more noble copper increased from 0.7% to 1.1%. This occurs because zinc and aluminum anodically dissolve, leaving behind copper inclusions that act as cathodic sites, accelerating corrosion. This highlights the need to store non-plated parts in a humidity-free environment to prevent even mild surface corrosion before plating.
Finding 2: The Critical "Die Cast Skin" and Hidden Internal Porosity
The die casting process creates a layered structure. The rapid solidification against the die walls forms a fine-grained, dense surface layer known as the "die cast skin," which is typically between 50 and 300 µm thick and is completely free of porosity. This skin is the ideal surface for plating.
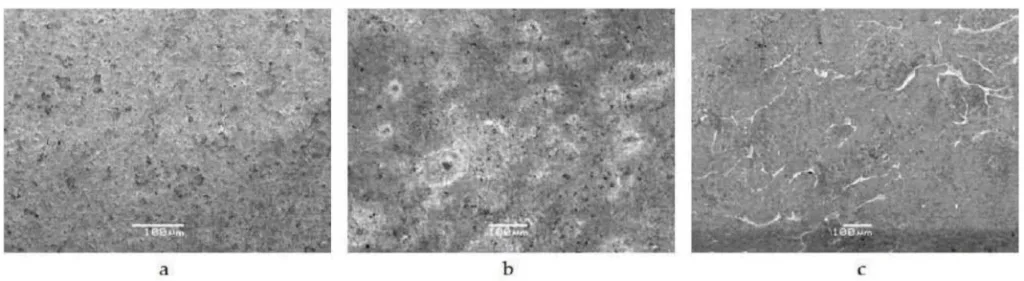
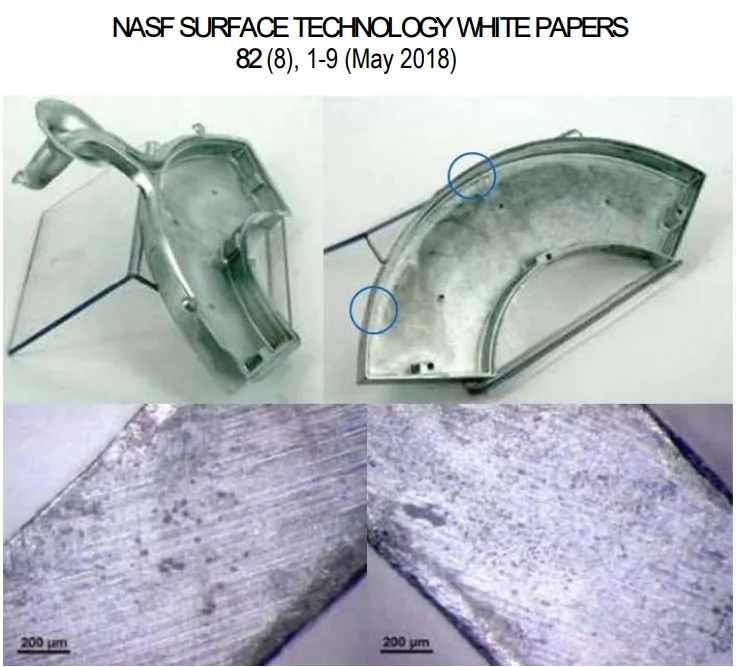
However, beneath this skin lies a porous inner core, as clearly shown in the light optical microscope image in Figure 4. This image shows the dense outer layer (approximately 80 µm) and the porous structure within the bulk material. Any post-casting operation, such as cutting off runners or aggressive grinding, that removes the skin will expose this porosity. These pores can trap plating solutions, leading to subsequent blistering, corrosion, and coating delamination.
Finding 3: Grinding is Essential but Must Be Controlled
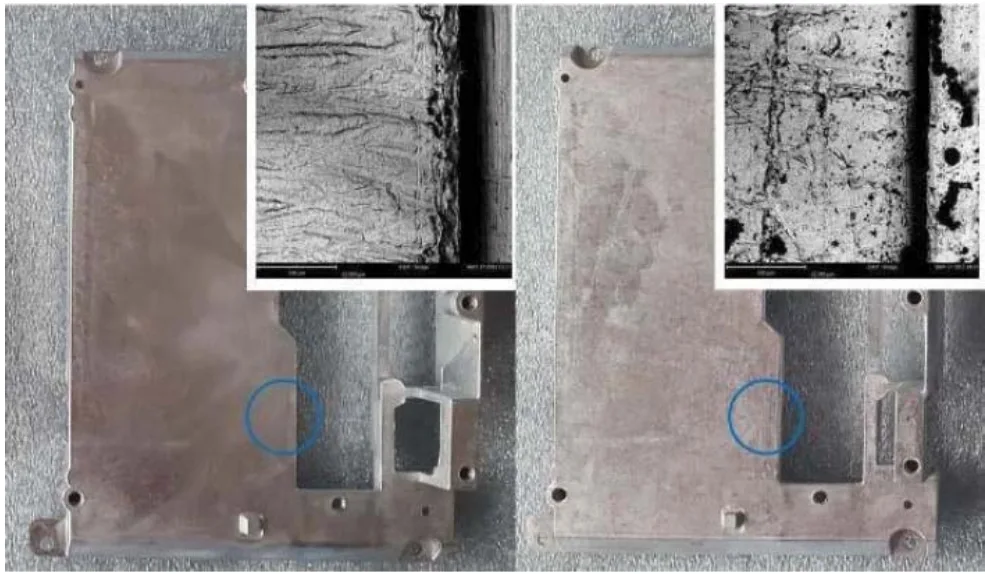
The surface of an as-cast part is not pure. It is often contaminated with aluminum oxide (due to aluminum's high affinity for oxygen) and residues from die lubricants. EDS analysis of an untreated surface (Table 3) showed elevated levels of aluminum (5.24%), carbon (6.06%), and oxygen (3.05%).
Grinding is a critical pretreatment step to remove these contaminants. As demonstrated in Table 4, after grinding, the surface composition changed dramatically: carbon and copper were reduced to 0.00%, and oxygen decreased to 1.99%. This creates a much cleaner surface for plating. However, the paper stresses that this mechanical treatment must be performed with particular attention to preserve the intact die cast skin to achieve the best results.
Practical Implications for R&D and Operations
- For Process Engineers: This study suggests that controlling the grinding process is a critical balance. The goal is to remove surface contaminants without penetrating the 50-300 µm die cast skin. It also reinforces that parts should be moved to plating immediately after wet pretreatment to prevent the onset of corrosion.
- For Quality Control Teams: The data in Figure 1 and Table 2 of the paper illustrates the effect of environmental exposure on the surface, which could inform new quality inspection criteria for stored, un-plated components. Furthermore, microscopic inspection of machined areas for exposed porosity (as seen in Figure 6) is a critical quality check before plating.
- For Design Engineers: The findings indicate that the placement of runner gates is a crucial design consideration. As noted on page 5, the runner gate should be placed far enough away from decoratively valuable areas to ensure that the removal process does not expose internal porosity on a critical surface.
Paper Details
Special Aspects of Electrodeposition on Zinc Die Castings
1. Overview:
- Title: Special Aspects of Electrodeposition on Zinc Die Castings
- Author: Valeriia Reveko and Per Møller
- Year of publication: 2018
- Journal/academic society of publication: NASF SURFACE TECHNOLOGY WHITE PAPERS
- Keywords: electrodeposition, zinc die cast, surface finishing, electroplating defects
2. Abstract:
Metal die casting has become a popular choice for the manufacturing of different consumer goods. Among the various engineering alloys and other metals, zinc alloys have often been preferred by manufacturers due to their qualities such as castability, dimensional stability and moderate solidification temperature, which provides energy and cost savings. Electroplating is a frequent choice to give zinc die cast components a high quality protective and decorative surface finish. However, applying this kind of treatment for die cast zinc components presents hidden challenges. In order to overcome these issues, a thorough morphology and composition analysis of die cast items was conducted. Special aspects of zinc die cast as a plating substrate are described and linked to the die casting process.
3. Introduction:
Currently, metal die casting has become a popular choice for the manufacturing of finished products. An increasing number of industrial producers are attracted by this highly economical and efficient process capable of providing near-net shaped components that fully satisfy the geometrical requirements. Zinc, as a basis material, offers a broad range of desirable properties, beginning with hardness, ductility, impact strength, self-lubricating characteristics, and exhibits excellent thermal and heat conductivity. Among the various engineering alloys and other metals, zinc alloys have often been preferred by manufacturers because of their castability, dimensional stability and moderate solidification temperatures, which provide significant energy and cost savings. Zinc die cast parts are characterized by high performance, low production price, recyclability (providing they are not nickel and chromium plated) and non-toxicity. This material is also highly regarded in the automotive industry. Despite this, some automotive manufacturers have started to pay increasing attention to polymers as an alternative to metals. However, zinc components remain irreplaceable in many applications, as reinforced polymers yet cannot match zinc cast alloys in strength and durability. Polymers are very attractive because the injection molding process used to form parts is cost-effective. Nonetheless, some of the physical properties of zinc, such as its high heat conductivity, make zinc indispensable and irreplaceable by polymers in fields where safety is an issue of utmost importance, such as car door handles.
4. Summary of the study:
Background of the research topic:
Zinc die casting is an economical and efficient manufacturing process for near-net shaped components. However, zinc's susceptibility to corrosion requires surface treatments like electroplating for protective and decorative finishes.
Status of previous research:
While the properties of zinc alloys and the principles of die casting are well-known, applying a high-quality electroplated finish consistently presents "hidden challenges." This study addresses the practical gap between the die casting process and the requirements for successful surface finishing.
Purpose of the study:
The purpose was to overcome the issues associated with electroplating on zinc die castings by conducting a thorough morphology and composition analysis of the cast items. The study aimed to describe the special aspects of zinc die cast as a plating substrate and link them directly to the die casting process.
Core study:
The core study involved the analysis of Zamak 5 zinc die cast samples. Researchers examined the material's susceptibility to corrosion via immersion testing, analyzed the surface composition and structure using SEM and EDS, investigated the formation of the "die cast skin" and internal porosity, and evaluated the effect of post-treatment grinding on surface purity before plating.
5. Research Methodology
Research Design:
The study employed an observational and analytical research design to characterize the surface and subsurface of zinc die cast components and evaluate the effects of corrosion and mechanical surface preparation.
Data Collection and Analysis Methods:
Data was collected using Scanning Electron Microscopy (SEM) for morphological imaging and Energy Dispersive X-ray Spectroscopy (EDS) for quantitative elemental analysis of the sample surfaces. Light optical microscopy was used for cross-sectional analysis.
Research Topics and Scope:
The research covered the impact of the alloy's chemical composition on corrosion, the microstructural features created by the die casting process (specifically the "die cast skin" and internal porosity), the role of process-related contaminants (such as release agents and oxides), and the effectiveness of grinding as a pre-plating treatment.
6. Key Results:
Key Results:
- Zinc alloys are susceptible to selective corrosion, where zinc and aluminum dissolve preferentially, increasing the surface concentration of more noble elements like copper, which can accelerate further corrosion.
- The die casting process creates a dual structure: a dense, non-porous "die cast skin" (50-300 µm thick) on the surface and a porous bulk material underneath.
- The "die cast skin" is the ideal surface for plating, but it can be compromised by machining or aggressive grinding, which exposes the underlying porosity.
- As-cast surfaces are contaminated with aluminum oxides, carbon, and other residues from die lubricants, which must be removed before plating.
- Grinding is an effective pretreatment that significantly reduces surface carbon and oxygen, but it must be controlled to maintain the integrity of the die cast skin.
Figure Name List:
- Figure 1 - SEM images of a zinc die cast sample surface (a) before and after immersion in a 0.5M sodium chloride solution for (b) 30 and (c) 60 minutes.
- Figure 2 - Zinc-aluminum binary phase diagram. The blue line shows the zinc-aluminum ratio in Zamak 5 alloy.
- Figure 3 - Light optical microscope image of a cross-section of the die cast sample. Light blue zinc-rich zones are surrounded by an aluminum-rich gray matrix.
- Figure 4 - Light optical microscope image of the die cast sample cross-section. The difference between the skin layer within the first 80 µm and the inner porous bulk is clearly visible.
- Figure 5 - Ellingham diagram for several metals giving the free energy of formation of metal oxides.
- Figure 6 - Photos illustrating the pores revealed after machining operations.
- Figure 7 - Cast items and abrasive chips in the working vessel and different types of abrasive chips (left) and SEM images of the surface of grinding chips (right).
- Figure 8 - Bowl vibrator for slide grinding at the die casting production site.
- Figure 9 - Macro photos and SEM images of a zinc die cast sample surface before (left) and after (right) grinding.

7. Conclusion:
The analysis of the impact of the material composition has revealed the necessity of storing non-treated zinc die cast parts under a controlled atmosphere in the absence of humidity, or better, to cover them with special protective greases in order to avoid mild corrosion of the surface. In this manner, the quality of the subsequent plating process is maintained within the product specifications. A recommended practice is to plate the zinc parts immediately after the pretreatment since wet conditions accelerate the corrosion process on the surface and hence affect the surface quality. Detailed consideration of the die casting process has demonstrated that reality always differs from expectations and very often the surface quality of components fails to live up to the requirements for plating. The presence of inner porosity in the bulk material, aluminum oxide on the outer layer and harmful impurities must not be ignored. The grinding process constitutes a valid pretreatment for plating, but as with all mechanical treatments, it must be held conscientiously in order to maintain the precious die cast skin, essential for the best results in plating and other subsequent treatments.
8. References:
- K. Miyoshi, "Solid Lubricants and Coatings for Extreme Environments: State-of-the-Art Survey," National Aeronautics and Space Administration, Springfield, 2007.
- ASTM B86-13, Standard Specification for Zinc and Zinc-Aluminum (ZA) Alloy Foundry and Die Castings, ASTM International, West Conshohocken, PA, 2013; www.astm.org.
- T. Savaşkan and M.Ş. Turhal, "Relationships between Cooling Rate, Copper Content and Mechanical Properties of Monotectoid-Based Zn-Al-Cu Alloys," Materials Characterization, 51 (4), 259-270 (2003).
- K.A. Esakul, "Intergranular Corrosion Failure in Zn-Al Alloy Solenoid Valve Seats," Handbook of Case Histories in Failure Analysis, ASM International, Materials Park, OH, Vol. 1, 1992.
- Z. Panossian and J.V. Ferrari, "Case Studies of Blistering Problems in Zinc-Plated Steel and Zinc-Plated Die-Cast Zinc Alloy Parts," Plating & Surface Finishing, 91 (9), 48-52 (2004).
- Landolt-Börnstein Database, Binary Systems.
- M. Gelfi, et al., "Microstructural and Mechanical Properties of Zinc Die Casting Alloys," Advanced Engineering Materials, 6 (10), 818-822 (2004).
- W.T. Andresen, Die Casting Engineering: A Hydraulic, Thermal and Mechanical Process, Marcel Dekker, New York, 2005.
- J. Titley, "The Role of Alloys and Melting Processes in the Cause and Elimination of Zinc Die Casting Defects," Global Casting Magazine, (2013).
- L.S. Aubrey, "Method for Filtering Molten Aluminum and Molten Aluminum Alloys," U.S. Patent 8,486,176 (July 16, 2013).
Expert Q&A: Your Top Questions Answered
Q1: Why was Zamak 5 chosen as the representative alloy for this study?
A1: The paper implies Zamak 5 was chosen because it is a popular and representative alloy of the Zamak class, which is preferred for its exceptional castability. The paper explains the function of its key alloying elements: aluminum improves fluidity, copper increases tensile strength and hardness, and magnesium provides resistance to intergranular corrosion. This makes it an excellent model for studying the complex surface interactions that affect plating.
Q2: The paper emphasizes the "die cast skin." What exactly is it and why is it so critical for electroplating?
A2: The "die cast skin" is a fine-grained, dense surface layer, typically 50 to 300 µm thick, that forms due to the rapid solidification of the molten zinc alloy against the relatively cool steel die walls. This layer is completely free of the porosity found in the bulk of the casting. It is critical because it provides a smooth, solid, and homogenous foundation for electroplating, which is essential for achieving strong adhesion and a defect-free decorative finish.
Q3: Table 2 shows the copper percentage increasing on the surface after the corrosion test. Is copper being added to the surface?
A3: No, copper is not being added. The data in Table 2 illustrates a phenomenon called selective corrosion. Zinc and aluminum are more electrochemically active (less noble) than copper. In the corrosive salt solution, the zinc and aluminum preferentially dissolve, leaving the more noble copper inclusions behind. As the surrounding material is removed, the relative concentration of copper on the remaining surface increases.
Q4: What is the primary source of the carbon contamination found on the as-cast surface (Table 3)?
A4: The paper indicates that the carbon contamination, along with other unexpected elements, is primarily from the die lubricant agents used during the casting process. These organic substances are present in the mold cavity and can thermally decompose during injection, leaving carbonaceous residues on the casting's surface. These residues act as a barrier to plating and must be removed.
Q5: The conclusion recommends plating parts immediately after pretreatment. Why is this delay so critical to avoid?
A5: This is critical because the pretreatment processes, such as grinding and chemical cleaning, leave the surface both wet and highly activated. As the paper states, "wet conditions accelerate the corrosion process on the surface." Any delay between pretreatment and plating allows for the onset of mild corrosion and oxidation on this activated surface, which degrades the surface quality and can lead to poor adhesion or other plating defects.
Q6: Can the internal porosity shown in Figure 4 be eliminated through process changes?
A6: The paper presents internal porosity as an inherent characteristic of the die casting process, resulting from the slower cooling and solidification that occurs in the bulk of the component compared to the surface. While process optimization can help manage and minimize it, the key takeaway is that it will likely be present. Therefore, the focus should be on process and design strategies—like proper gate location and controlled mechanical finishing—that avoid exposing this porosity on critical surfaces.
Conclusion: Paving the Way for Higher Quality and Productivity
The challenge of achieving a perfect electroplated finish on zinc die castings is not a simple matter of cleaning; it is a complex interplay between alloy chemistry, solidification physics, and post-casting treatments. This research by Reveko and Møller demystifies the process by highlighting the critical importance of the "die cast skin" and the dangers of hidden porosity and surface contamination. By understanding these factors, manufacturers can move from reactive problem-solving to proactive process control, ensuring a higher quality Zinc Die Casting Surface Finish.
At CASTMAN, we are committed to applying the latest industry research to help our customers achieve higher productivity and quality. If the challenges discussed in this paper align with your operational goals, contact our engineering team to explore how these principles can be implemented in your components.
Copyright Information
- This content is a summary and analysis based on the paper "Special Aspects of Electrodeposition on Zinc Die Castings" by "Valeriia Reveko and Per Møller".
- Source: NASF SURFACE TECHNOLOGY WHITE PAPERS, 82 (8), 1-9 (May 2018).
This material is for informational purposes only. Unauthorized commercial use is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.