This article introduces the paper 'Eutectic Phase Investigation in a Ca-added AM50 Magnesium Alloy Produced by Die Casting' published by 'The Japan Institute of Metals'.
1. Overview:
- Title: Eutectic Phase Investigation in a Ca-added AM50 Magnesium Alloy Produced by Die Casting
- Author: Yoshihiro Terada, Naoya Ishimatsu, Yukako Mori and Tatsuo Sato
- Publication Year: 2005
- Publishing Journal/Academic Society: Materials Transactions, The Japan Institute of Metals
- Keywords: magnesium alloy, die-cast, phase identification, Al₂Ca, magnesium-aluminum-calcium ternary system
2. Abstracts or Introduction
The eutectic phase within a 1.72 mass pct calcium added AM50 die-cast alloy, homogenized at 673 K, was investigated utilizing X-ray Diffraction (XRD) and Energy Dispersive Spectrometry (EDS). Experimental results from XRD and EDS revealed that the eutectic phase is composed of Al₂Ca phase exhibiting the C15 structure and incorporates 10.76 atomic pct magnesium in its equilibrium state. The solubility lobe of the Al₂Ca phase is oriented parallel to the equi-66.7 at% Al composition line within the Mg-Al-Ca ternary system. This orientation suggests a preferential substitution of magnesium for calcium within the Al₂Ca phase.
3. Research Background:
Background of the Research Topic:
Magnesium alloys are recognized for their low density among conventional engineering metals, making them attractive for automotive applications to reduce weight and enhance fuel efficiency. While automotive applications are expanding, their current usage is primarily limited to components operating at room temperature, such as instrument panels and steering wheels. To broaden the application of magnesium alloys, particularly for powertrain components like transmission cases and engine blocks that operate at temperatures up to 450 K, enhancing their high-temperature performance is crucial. Calcium is considered as a cost-effective and lightweight alternative to rare-earth elements for improving the high-temperature mechanical properties of Mg-Al alloys.
Status of Existing Research:
Prior research has demonstrated that adding 1.72 mass pct calcium to the die-cast AM50 alloy results in a thousandfold increase in creep strength. AM50 alloy is already recognized for its favorable combination of die-castability, ductility, and fracture toughness among commercial magnesium alloys. The enhanced creep resistance in calcium-added AM50 alloy is attributed to the eutectic phase surrounding the α-Mg grains. This eutectic phase is believed to play a critical role in grain boundary strengthening and resisting plastic flow of the α-Mg grains during creep deformation.
Necessity of the Research:
Understanding the nature of the eutectic phase in Ca-added AM50 alloy is crucial for optimizing its high-temperature performance. Non-equilibrium phases can arise in as die-cast Mg-Al-Ca alloys. To accurately identify the equilibrium eutectic phase, isothermal homogenization treatment is necessary. This study aims to identify the eutectic phase formed in a 1.72 mass pct calcium added AM50 die-cast alloy after homogenization treatment to ensure the alloy reaches an equilibrium state for phase identification.
4. Research Purpose and Research Questions:
Research Purpose:
The primary objective of this research is to identify the eutectic phase formed in a 1.72 mass pct calcium added AM50 die-cast alloy. This identification is achieved through a combination of X-ray Diffraction (XRD) and Energy Dispersive Spectrometry (EDS) techniques, applied to a homogenized sample to ensure equilibrium conditions.
Key Research:
The key research focus is on characterizing the eutectic phase in the homogenized AM50-1.72 mass%Ca die-cast alloy. This involves:
- Determining the crystal structure of the eutectic phase using XRD.
- Identifying the elemental composition of the eutectic phase using EDS.
- Comparing the eutectic phase in the homogenized alloy with that in the as die-cast alloy to understand the phase evolution towards equilibrium.
- Analyzing the position of the eutectic phase composition in the Mg-Al-Ca ternary phase diagram to understand the substitution behavior of elements within the phase.
Research Hypotheses:
The study hypothesizes that the eutectic phase in the 1.72 mass pct calcium added AM50 die-cast alloy, after homogenization at 673 K, will be an equilibrium phase, potentially Al₂Ca based on previous indications. It is also hypothesized that magnesium may substitute into the Al₂Ca phase, and the extent and preference of this substitution will be investigated by analyzing the solubility lobe direction in the Mg-Al-Ca ternary system.
5. Research Methodology
Research Design:
This research employs an experimental design focused on phase identification and characterization. The study involves material processing (die-casting and homogenization), microstructural observation, and phase analysis using advanced analytical techniques.
Data Collection Method:
The data collection methods include:
- Die Casting: AM50 alloy with 1.72 mass pct calcium addition (Mg-4.98Al-1.72Ca-0.29Mn in mass pct) was produced using a cold chamber die-casting machine.
- Homogenization: Die-cast materials were homogenized at 673 K for 36 ks followed by water quenching to achieve equilibrium state.
- Microscopy: Microstructure observation was performed using Field Emission Scanning Electron Microscopy (FE-SEM) at 5 kV.
- X-ray Diffraction (XRD): Phase identification was conducted using XRD with CuKα radiation, scanning angles of 20° < 2θ < 80°, and a 0.02° step scan.
- Energy Dispersive Spectrometry (EDS): Compositional analysis of the phases was performed using EDS attached to a Transmission Electron Microscope (TEM) operated at 200 kV.
Analysis Method:
The analysis methods include:
- XRD Pattern Analysis: Identification of crystal structures by comparing experimental XRD spectra with JCPDS card data for expected phases (α-Mg, Mg₁₇Al₁₂, Mg₂Ca, Al₂Ca). Fitting the experimental XRD result to reference spectra to confirm phase presence.
- EDS Compositional Analysis: Quantitative analysis of elemental composition (Mg, Al, Ca) in different phases observed in SEM and TEM.
- Phase Diagram Analysis: Plotting the composition of the eutectic phase on the Mg-Al-Ca ternary phase diagram to analyze the solubility behavior and elemental substitution within the eutectic phase, particularly in relation to the Al₂Ca phase.
Research Subjects and Scope:
The research subject is a 1.72 mass pct calcium added AM50 magnesium alloy produced by die casting. The scope is limited to the identification and characterization of the eutectic phase in this specific alloy composition after homogenization at 673 K for 36 ks. The study focuses on phase identification, crystal structure determination, and compositional analysis of the eutectic phase using XRD, EDS, and SEM/TEM techniques.
6. Main Research Results:
Key Research Results:
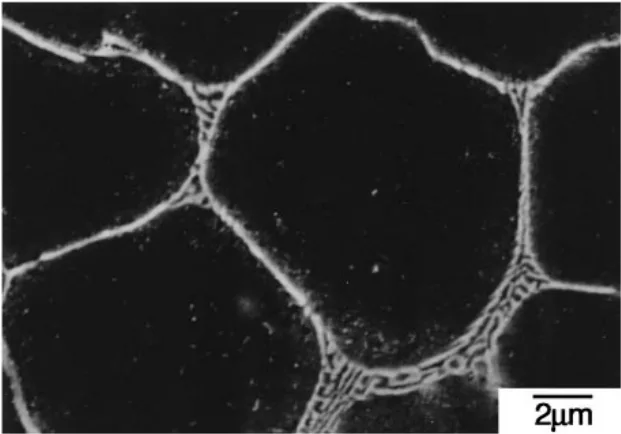
- Eutectic Phase Morphology: Homogenization treatment at 673 K transformed the eutectic phase morphology from a grain boundary network in the as-die-cast condition (Fig. 1) to a spherical shape (Fig. 2). The α-Mg grain size remained approximately constant at 5.3 µm after homogenization.
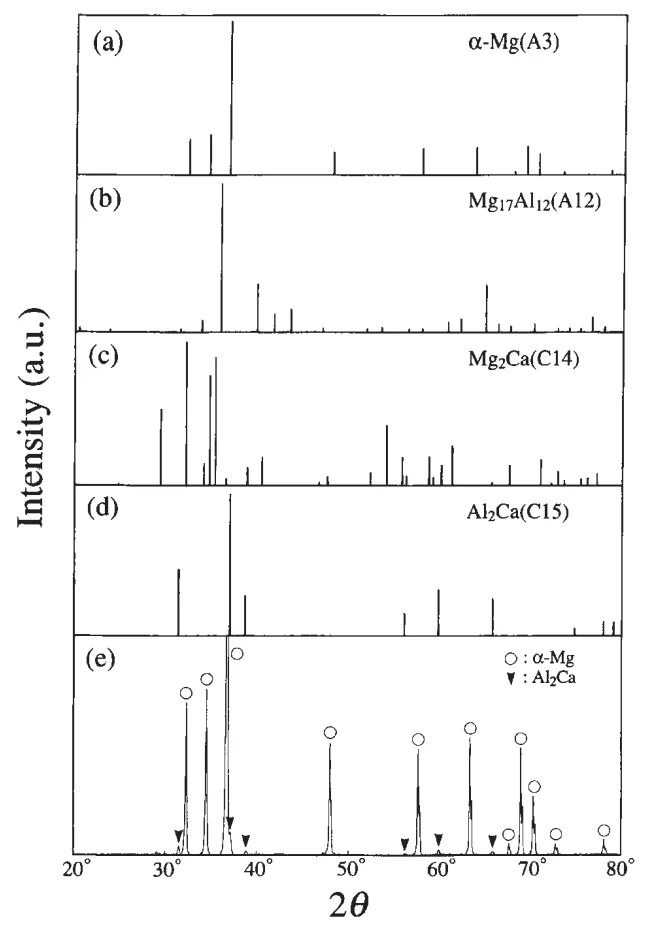
- Phase Identification by XRD: XRD analysis (Fig. 3) of the homogenized alloy confirmed the presence of two phases: α-Mg phase with A3 structure and Al₂Ca phase with C15 structure. This identified the eutectic phase as Al₂Ca in the equilibrium state.
- Compositional Analysis by TEM/EDS: EDS analysis (Table 1) revealed that the α-Mg phase in the homogenized alloy contains 3.55 atomic pct aluminum and 0.18 atomic pct calcium. The Al₂Ca eutectic phase in the homogenized alloy contains 10.76 atomic pct magnesium, 66.66 atomic pct aluminum, and 22.35 atomic pct calcium. In comparison, the eutectic phase in the as-die-cast alloy contained 17.47 atomic pct magnesium, 59.80 atomic pct aluminum, and 22.87 atomic pct calcium.
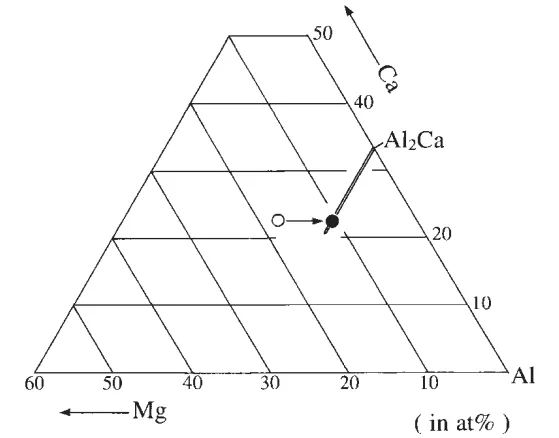
- Solubility Lobe Direction: Plotting the eutectic phase composition in the Mg-Al-Ca ternary grid (Fig. 4) showed that the composition shift due to homogenization occurs along the equi-Ca composition line and reaches the equi-66.7 at%Al composition line. The solubility lobe of the Al₂Ca phase is parallel to the equi-66.7 at%Al composition line, indicating magnesium preferentially substitutes the calcium site in the Al₂Ca phase.
Analysis of presented data:
- Microstructural Change: Figure 2 shows the FE-SEM micrograph of the eutectic phase after homogenization, demonstrating a change in morphology to spherical particles compared to the as-die-cast state in Figure 1.
- XRD Spectra Interpretation: Figure 3 presents XRD spectra confirming the presence of α-Mg and Al₂Ca phases in the homogenized alloy. The experimental spectrum (e) is consistent with the combined spectra of α-Mg (a) and Al₂Ca (d).
- Compositional Shift: Table 1 shows a decrease in magnesium content in the Al₂Ca phase after homogenization, indicating a shift towards equilibrium composition. Figure 4 visually represents this compositional shift in the ternary phase diagram, highlighting the movement along the equi-Ca line and the final position on the equi-66.7 at%Al line.
Figure Name List:
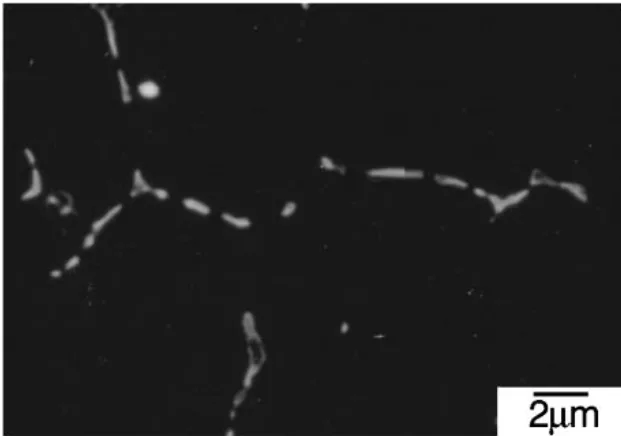

- Fig. 1 FE-SEM micrograph of the eutectic phase formed in the AM50-1.72 mass%Ca die-cast alloy.
- Fig. 2 FE-SEM micrograph of the eutectic phase in the AM50-1.72 mass%Ca die-cast alloy homogenized at 673 K for 36 ks.
- Fig. 3 X-ray diffraction spectra for four kinds of phases expected to appear in the AM50-1.72 mass%Ca die-cast alloy homogenized at 673 K for 36 ks ((a)-(d)). The scattering angle and intensity for each phase are derived from the JCPDS card data. The experimentally obtained XRD spectrum for the homogenized alloy is shown in (e).
- Fig. 4 Composition of the Al₂Ca phase appeared in the AM50-1.72 mass%Ca die-cast alloy homogenized at 673 K for 36 ks (solid symbol), together with that of the eutectic phase in the as die-cast alloy (open symbol).
- Fig. 5 Direction of solubility lobes of the ternary Ni₃Al phase at 1273 K reported by Ochiai et al.²⁹)
7. Conclusion:
Summary of Key Findings:
This study successfully identified the eutectic phase in a 1.72 mass pct calcium added AM50 die-cast magnesium alloy after homogenization at 673 K for 36 ks. The eutectic phase was determined to be Al₂Ca with a C15 structure, containing 10.76 atomic pct magnesium in its equilibrium state. The solubility lobe of the Al₂Ca phase in the Mg-Al-Ca ternary system is parallel to the equi-66.7 at%Al composition line, indicating that magnesium preferentially substitutes for calcium in the Al₂Ca phase.
Academic Significance of the Study:
This research provides a fundamental understanding of the phase equilibrium in the Mg-Al-Ca ternary system, specifically regarding the Al₂Ca phase in calcium-added AM50 magnesium alloy. The identification of the eutectic phase as Al₂Ca with magnesium substitution and the determination of the solubility lobe direction contribute to the fundamental knowledge of ternary phase diagrams and elemental substitution behavior in intermetallic compounds. This understanding is crucial for alloy design and property prediction in Mg-Al-Ca systems.
Practical Implications:
The identification of the equilibrium eutectic phase in Ca-added AM50 alloy has practical implications for optimizing the alloy's high-temperature creep resistance. Understanding the composition and stability of the Al₂Ca phase allows for better control of microstructure through heat treatments and alloy composition adjustments. This can lead to the development of improved die-cast magnesium alloys for elevated temperature applications, particularly in the automotive industry for powertrain components.
Limitations of the Study and Areas for Future Research:
This study focused on a single alloy composition (AM50-1.72 mass%Ca) and a specific homogenization temperature (673 K). The solubility limit of magnesium in the Al₂Ca phase was not fully clarified. Future research should investigate:
- The solubility limit of magnesium in the Al₂Ca phase in more detail across a range of temperatures and alloy compositions.
- The effect of different homogenization temperatures and times on the eutectic phase evolution and microstructure.
- The mechanical properties, particularly creep behavior, of the homogenized alloy in relation to the identified equilibrium eutectic phase.
- The phase diagram of the Al-rich corner of the Mg-Al-Ca ternary system to further elucidate the stability and compositional range of the Al₂Ca phase.
8. References:
- 1) S. Das: JOM 55 (2003) 22–26.
- 2) B. L. Mordike and T. Ebert: Mater. Sci. Eng. A302 (2001) 37–45.
- 3) G. S. Cole: Mater. Sci. Forum 419–422 (2003) 43–50.
- 4) T. Kaneko and M. Suzuki: Mater. Sci. Forum 419–422 (2003) 67–72.
- 5) S. Schumann and H. Friedrich: Mater. Sci. Forum 419–422 (2003) 51–56.
- 6) E. Aghion, B. Bronfin, F. V. Buch, S. Schumann and H. Friedrich: JOM55 (2003) 30–33.
- 7) M. O. Pekguleryuz and A. A. Kaya: Adv. Eng. Mater. 5 (2003) 866–878.
- 8) P. Bakke and H. Westengen: Adv. Eng. Mater. 5 (2003) 879–885.
- 9) A. A. Luo, M. P. Balogh and B. R. Powell: Metall. Mater. Trans. A 33A(2002) 567–574.
- 10) K. Maruyama, M. Suzuki and H. Sato: Metall. Mater. Trans. A 33A(2002) 875–882.
- 11) E. Baril, P. Labelle and M. O. Pekguleryuz: JOM 55 (2003) 34–39.
- 12) I. P. Moreno, T. K. Nandy, J. W. Jones, J. E. Allison and T. M. Pollock:Scr. Mater. 48 (2003) 1029–1034.
- 13) H. Gjestland, G. Nussbaum, G. Regazzoni, O. Lohne and O. Bauger:Mater. Sci. Eng. A134 (1991) 1197–1200.
- 14) R. Ninomiya, T. Ojiro and K. Kubota: Acta Metall. Mater. 43 (1995)669–674.
- 15) D. Wenwen, S. Yangshan, M. Xuegang, X. Feng, Z. Min and W.Dengyun: Mater. Sci. Eng. A356 (2003) 1–7.
- 16) Y. Terada, N. Ishimatsu, R. Sota, T. Sato and K. Ohori: Mater. Sci.Forum 419–422 (2003) 459–464.
- 17) Y. Terada, R. Sota, N. Ishimatsu, T. Sato and K. Ohori: Metall. Mater.Trans. A 35A (2004) 3029–3032.
- 18) I. J. Polmear: Mater. Sci. Technol. 10 (1994) 1–16.
- 19) K. Ozturk, Y. Zhong, A. A. Luo and Z. K. Liu: JOM 55 (2003) 40–44.
- 20) A. Suzuki, N. D. Saddock, J. W. Jones and T. M. Pollock: Scr. Mater.51 (2004) 1005–1010.
- 21) J. Grobner, D. Kevorkov, I. Chumak and R. Schmid-Fetzer: Z.Metallkd. 94 (2003) 976–982.
- 22) T. B. Massalski: Binary Alloy Phase Diagrams, 2nd edn., (ASM International, Materials Park, 1990).
- 23) H. Okamoto: Desk Handbook Phase Diagrams for Binary Alloys,(ASM International, Materials Park, 2000).
- 24) D. Kevorkov and R. Schmid-Fetzer: Z. Metallkd. 92 (2001) 946–952.
- 25) G. Petzow and G. Effenberg: Ternary Alloys, (VCH, Weinheim, 1988).
- 26) P. Villars, A. Prince and H. Okamoto: Handbook of Ternary Alloy Phase Diagrams, (ASM International, Materials Park, 1995).
- 27) D. L. Anton: Intermetallic Compounds, vol. 2, Practice, ed. by J. H. Westbrook and R. L. Fleischer (Wiley, New York, 1994) pp. 3.
- 28) C. T. Liu and D. P. Pope: Intermetallic Compounds, vol. 2, Practice, ed. by J. H. Westbrook and R. L. Fleischer (Wiley, New York, 1994) pp. 17.
- 29) S. Ochiai, Y. Oya and T. Suzuki: Acta Metall. 32 (1984) 289–298.
9. Copyright:
- This material is "The Japan Institute of Metals"'s paper: Based on "Eutectic Phase Investigation in a Ca-added AM50 Magnesium Alloy Produced by Die Casting".
- Paper Source: doi:10.4028/www.scientific.net/MSF.488-489.931
This material was summarized based on the above paper, and unauthorized use for commercial purposes is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.