Increased Iron in AlSi7Mg0.3: A Corrosion Risk or a Cost-Saving Opportunity?
This technical summary is based on the academic paper "Analysis of the Increased Iron Content on the Corrosion Resistance of the AlSi7Mg0.3 Alloy Casting" by Jaroslava Svobodova, Milan Lunak, and Michal Lattner, published in Manufacturing Technology (2019). It has been analyzed and summarized for technical experts by CASTMAN.
![Fig. 1 Interdendritic spaces of the AlSi12NiMgCu alloy filled with rough phases Al5FeSi (Fe content 0.82 [wt. %]) [9]](https://castman.co.kr/wp-content/uploads/image-3046.webp)
Keywords
- Primary Keyword: AlSi7Mg0.3 Corrosion Resistance
- Secondary Keywords: Iron Contamination Aluminum, Alloy Casting Defects, Salt Spray Testing, Secondary Aluminum Alloys
Executive Summary
- The Challenge: The increasing use of recycled aluminum raises iron content in alloys like AlSi7Mg0.3, creating widespread concern about its negative impact on corrosion resistance.
- The Method: Two AlSi7Mg0.3 castings, one with standard iron (0.169 wt. %) and one with elevated iron (0.319 wt. %), were subjected to a 480-hour salt spray test and evaluated using confocal laser microscopy.
- The Key Breakthrough: Microscopic analysis revealed no significant difference in corrosion resistance between the standard and high-iron castings under the specified test conditions.
- The Bottom Line: An increased iron content of up to 0.319 wt. % does not necessarily degrade the corrosion resistance of AlSi7Mg0.3, potentially allowing for greater use of cost-effective secondary aluminum feedstock.
The Challenge: Why This Research Matters for HPDC Professionals
In the high-pressure die casting industry, balancing cost and performance is paramount. Al-Si based alloys are workhorses, especially in the automotive sector, due to their excellent mechanical and foundry properties. However, the push towards sustainability and cost reduction through recycled (secondary) aluminum introduces a significant challenge: iron contamination.
Iron is a common and difficult-to-remove impurity that can enter the melt from scrap, handling tools, or feedstock. It is widely believed that elevated iron levels lead to the formation of brittle, needle-like intermetallic phases (e.g., Al5FeSi), which can degrade mechanical properties and, crucially, compromise corrosion resistance. This forces manufacturers to either dilute melts with expensive pure aluminum or reject feedstock, increasing costs. This study directly addresses the critical question: at what point does increased iron content actually begin to harm the corrosion performance of AlSi7Mg0.3, one of the industry's most widely used alloys?
The Approach: Unpacking the Methodology
To provide a clear, industry-relevant answer, the researchers conducted a controlled experiment comparing two sets of castings.
- Material: AlSi7Mg0.3 (EN AC-42100), a hypoeutectic aluminum alloy.
- Samples: Two castings intended for use as car washer parts were produced via low-pressure casting into a metal mold and subjected to a T6 heat treatment (annealing and artificial aging).
- Casting 1 (Control): Contained a standard iron level of 0.169 wt. %.
- Casting 2 (Test): Contained an increased iron level of 0.319 wt. %.
- Corrosion Test: The castings were placed in a corrosion chamber for a 480-hour salt spray test, following the ČSN EN ISO 9227 standard. The test environment consisted of a 5% NaCl salt mist at a temperature of 50°C.
- Analysis: The surface condition of the samples before and after the corrosion test was evaluated using an Olympus LEXT OLS 3100 confocal laser microscope, allowing for detailed microscopic analysis of any corrosion attack.
The Breakthrough: Key Findings & Data
The experiment yielded surprising results that challenge conventional assumptions about iron's impact on corrosion.
Finding 1: Increased Iron Content Did Not Worsen Corrosion Resistance
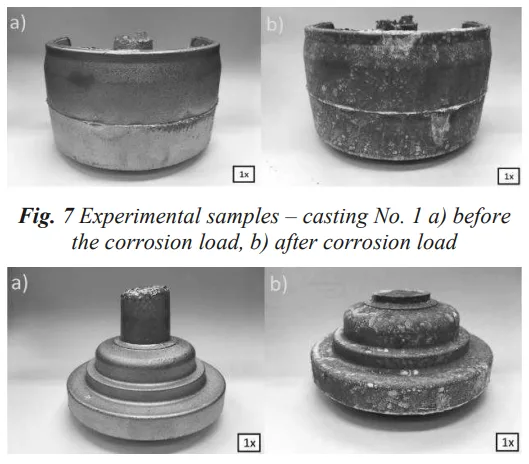
Despite one casting having nearly double the iron content of the other, the study found no evidence of worsened corrosion. The paper's conclusion is definitive: "The results of the microscopic analysis did not show any significant influence of the increased Fe content on the corrosion resistance of the casting compared to the casting in which Fe was normal." The photographic documentation in Figures 7 and 8 of the paper, showing the castings before and after the 480-hour test, visually supports this finding, with both samples exhibiting similar surface changes.
Finding 2: Absence of Detrimental Iron-Based Intermetallic Phases
A key concern with high iron is the formation of specific intermetallic phases that can act as cathodic sites and accelerate localized corrosion. However, the microscopic analysis in this study did not identify the presence of these harmful phases. The paper states, "in the material structure, this increased Fe content did not manifest in phases that would indicate increased Fe content." This suggests that at a concentration of 0.319 wt. %, the iron remained distributed in the microstructure in a way that did not negatively influence the alloy's passive state or its response to the corrosive salt spray environment. The primary surface defects observed on both samples were common interdendritic porosity, not defects induced by iron.
Practical Implications for R&D and Operations
- For Materials & Sourcing Teams: This study suggests that feedstock with slightly elevated iron levels (up to 0.319 wt. %) may not need to be rejected solely on the basis of corrosion concerns for similar applications. This could expand the pool of acceptable secondary aluminum, leading to significant cost savings.
- For Quality Control Teams: The data in the paper illustrates that for this alloy and Fe level, standard surface porosity (as seen in Figures 11, 13, and 18) remains the primary focus for inspection. The increased iron did not introduce new, corrosion-accelerating microstructural defects that would require new inspection criteria.
- For Design Engineers: The findings indicate that for components where corrosion is a key design criterion, specifying an upper iron limit around 0.3 wt. % in AlSi7Mg0.3 may be acceptable for certain environments, offering potential cost savings without compromising performance.
Paper Details
Analysis of the Increased Iron Content on the Corrosion Resistance of the AlSi7Mg0.3 Alloy Casting
1. Overview:
- Title: Analysis of the Increased Iron Content on the Corrosion Resistance of the AlSi7Mg0.3 Alloy Casting
- Author: Jaroslava Svobodova, Milan Lunak, Michal Lattner
- Year of publication: 2019
- Journal/academic society of publication: MANUFACTURING TECHNOLOGY
- Keywords: AlSi7Mg0.3, Increased iron content, Corrosion resistance, Microscopic analysis
2. Abstract:
Aluminium alloys are, due to their properties, an important part of all shaped castings made especially for the aerospace and automotive industry. One of the widely used alloys, for the production of castings in the automotive industry, is the AlSi7Mg0.3 alloy. In the experiment, which is the content of this paper, two castings from AlSi7Mg0.3 alloy were tested. Iron is a common contaminant in aluminium alloys and its content in the alloy is increased by the use of secondary aluminium alloys or by various causes during the production process itself. In the experiment, the corrosion resistance of castings was evaluated (in one of them the increased Fe content) under the specified conditions in the corrosion chamber with salt mist. Corrosion properties were evaluated using confocal laser microscopy. Based on the results of the microscopic analysis, the relevant conclusions were formulated. From the experiment, it is evident that the increased iron content (0.319 [wt. %]) does not, in this case, affect the corrosion resistance of casting.
3. Introduction:
Al-Si based alloys are representatives of the most widely used types of foundry aluminium alloys, finding application in the automotive, aerospace, and food industry. Their properties are greatly influenced by chemical composition. Elements are either intentionally added (alloying elements) or are undesirable (admixtures). Iron is a common undesirable element, often introduced through the remelting of aluminum scrap. The increasing pressure to recycle waste is leading to higher proportions of unwanted elements in aluminum alloys. Suppressing the adverse effect of iron is a considerable problem; the most common method is diluting the melt with pure aluminum, which is expensive. Iron has low solubility and forms intermetallic phases like Al5FeSi, which can reduce the plastic properties of the material.
4. Summary of the study:
Background of the research topic:
The properties of Al-Si aluminum alloys are heavily dependent on their chemical composition, particularly the presence of impurities like iron. Iron is a common contaminant, and its content is rising due to increased recycling. High iron content is known to form brittle intermetallic phases that can degrade mechanical and corrosion properties.
Status of previous research:
Previous research has established that iron above certain thresholds (e.g., 0.6 wt. %) leads to the formation of brittle Al5FeSi phases. The general understanding is that impurities reduce corrosion resistance. However, the precise effect of moderately increased iron levels on the corrosion performance of AlSi7Mg0.3 under specific, standardized test conditions required further practical investigation.
Purpose of the study:
The aim of this paper was to experimentally verify and evaluate the corrosion resistance of AlSi7Mg0.3 alloy castings with an increased iron content compared to a casting with a standard iron content.
Core study:
The core of the study involved subjecting two AlSi7Mg0.3 castings—one with 0.169 wt. % Fe and another with 0.319 wt. % Fe—to a 480-hour salt spray corrosion test. The surfaces of the castings were then analyzed using confocal laser microscopy to determine if the higher iron content led to a measurable increase in corrosion attack.
5. Research Methodology
Research Design:
The study used a comparative experimental design. Two sample groups of AlSi7Mg0.3 castings, differing only in their iron content, were subjected to identical corrosive conditions. The surface state before and after the test was analyzed to isolate the effect of the increased iron content.
Data Collection and Analysis Methods:
- Chemical Composition: An optical emission spectrometer (Q4 Tasman) was used to determine the exact chemical composition of the castings (Tables 2 and 3).
- Corrosion Test: A standardized salt spray test was conducted according to ČSN EN ISO 9227 (480 hours, 5% NaCl solution, 50°C).
- Microscopic Analysis: An Olympus LEXT OLS 3100 confocal laser microscope was used for metallographic analysis and evaluation of the surface condition before and after corrosion loading. Image analysis was used to evaluate the sample surfaces.
Research Topics and Scope:
The research was focused specifically on the AlSi7Mg0.3 alloy. The scope was limited to evaluating the effect of an iron content of 0.319 wt. % on corrosion resistance under salt spray test conditions. The study did not evaluate mechanical properties or other forms of corrosion.
6. Key Results:
Key Results:
- The increased iron content of 0.319 wt. % did not have a significant influence on the corrosion resistance of the AlSi7Mg0.3 casting when compared to a casting with a standard Fe content of 0.169 wt. %.
- The increased iron content did not manifest in the formation of detrimental intermetallic phases that would typically be associated with poor corrosion performance.
- Microscopic analysis identified common interdendritic porosity on the surface of both samples, but no other defects attributable to the higher iron content were observed.
Figure Name List:
- Fig. 1 Interdendritic spaces of the AlSi12NiMgCu alloy filled with rough phases Al5FeSi (Fe content 0.82 [wt. %]) [9]
- Fig. 2 Basic types of specific corrosion attack of Al alloys [4]
- Fig. 3 Pourbaix diagram for aluminium
- Fig. 4 Dependence of Al corrosion rate on temperature and HNO3 concentration [4]
- Fig. 5 Dependence of Al corrosion rate on temperature and H2SO4 concentration [4]
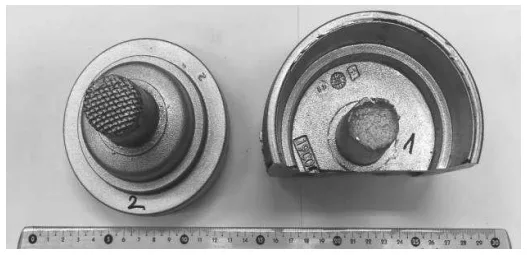
- Fig. 6 Experimental samples of AlSi7Mg0.3 castings
- Fig. 7 Experimental samples – casting No. 1 a) before the corrosion load, b) after corrosion load
- Fig. 8 Experimental samples – casting No. 2 a) before the corrosion load, b) after corrosion load
- Fig. 9 Casting No. 1 structure
- Fig. 10 Casting No. 1 surface before the corrosion load
- Fig. 11 Casting No. 1 surface before the corrosion load
- Fig. 12 Casting No. 1 surface after the corrosion load
- Fig. 13 Casting No. 1 surface after the corrosion load
- Fig. 14 Casting No. 2 structure
- Fig. 15 Casting No. 2 surface before the corrosion load
- Fig. 16 Casting No. 2 surface before the corrosion load
- Fig. 17 Casting No. 2 surface after the corrosion load
- Fig. 18 Casting No. 2 surface after the corrosion load
![Fig. 2 Basic types of specific corrosion attack of Al alloys [4]](https://castman.co.kr/wp-content/uploads/image-3047.webp)
7. Conclusion:
The samples were subjected to corrosion loading in a salt mist chamber according to ČSN EN ISO 9227. The results of the microscopic analysis did not show any significant influence of the increased Fe content on the corrosion resistance of the casting compared to the casting with normal Fe content. Also, in the material structure, this increased Fe content did not manifest in phases that would indicate increased Fe content. It can be stated that the effect of higher Fe content (0.319 [wt. %]) on the corrosion resistance of the casting was not proved by microscopic analysis of the casting surface.
8. References:
- [1] KOLEKTIV ČESKOSLOVENSKÝCH MAĎARSKÝCH NĚMECKÝCH A POLSKÝCH AUTORŮ (1969). Příručka o hliníku. Praha 1: SNTL
- [2] MICHNA, Š., MICHNOVÁ, L. (2014). Neželezné kovy. Ústí n/L: PrintPoint Praha, s.r.o.
- [3] MICHNA, Š., LUKÁČ, I., OČENÁČEK, V., KOŘENÝ, R., DRÁPALA, J., SCHNEIDER, H., MIŠKUFOVÁ, A., a kol. (2005). Encyklopedie hliníku. Prešov, Adin, s.r.o., ISBN 80-89041-4
- [4] KOCICH, J. TULEJA, S. (1989). Korózia a ochrana kovov. Alfa – Vydavatel'stvo technickej a ekonomickej literatúry Bratislava.
- [5] MONDOLFO L. F. (1976). Aluminum alloys: structure and properties. London, London; Boston: Butterworths, ISBN 04-08706-80-5
- [6] DAVIS, J., R. (1999). Corrosion of Aluminum and Aluminum Alloys. ASM International
- [7] VOJTĚCH, D. (2006). Kovové materiály. KANAG-TIST, s.r.o., VŠCHT Praha
- [8] BOLIBRUCHOVÁ, D., TILLOVÁ, E. (2005). Zlievarenské zliatiny Al-Si. Žilina: EDIS – vydavatel'stvo ŽU
- [9] HREN, I., SVOBODOVA, J., MICHNA. Š. (2018). Influence of Al5FeSi phases on the cracking of castings at Al-Si alloys. Archives of Foundry Engineering, Vol. 18, No. 4, pp. 120-124
- [10] VOJTĚCH, D., KUČERA, V. (2017). Influence of the Heat Treatment on Corrosion Behaviour and Mechanical Properties of the AA 7075 Alloy. Manufacturing Technology, Vol. 17, No. 5, pp. 747-752
- [11] FOUSOVÁ, M., DVORSKÝ, D., VOJTĚCH, D. (2017). Corrosion Properties of AlSi10Mg Alloy Prepared by Gravity Casting and 3D Printing Technology. Manufacturing Technology, Vol. 17, No. 6, pp. 847-853
- [12] BOLIBRUCHOVÁ, D., BRŮNA, M. (2017). Impact of the Elements Affecting the Negative Iron-Based Phases Morphology in Aluminium Alloys - Summary Results. Manufacturing Technology, Vol. 17, No. 5, pp. 675-679
- [13] PODPROCKÁ, R., BOLIBRUCHOVÁ, D. (2018). The Role of Manganese in the Alloy Based on Al-Si-Mg with Higher Iron Content. Manufacturing Technology, Vol. 18, No. 4, pp. 650-654
- [14] ČSN 42 4332 Slitina hliníku na odlitky 42 4332 AlSi7Mg(Fe)
- [15] ČSN EN ISO 9227 (038132) Korozní zkoušky v umělých atmosférách - Zkoušky solnou mlhou
Expert Q&A: Your Top Questions Answered
Q1: Why was a 480-hour salt spray test chosen for this experiment?
A1: The paper states that the 480-hour load time was a "customer requirement for which castings were made." The experimental samples were actual parts for a car washer, so this test duration reflects a specific, real-world qualification standard for the component's end-use environment, making the results highly relevant for industrial applications.
Q2: The introduction mentions brittle Al5FeSi phases forming above 0.6 wt. % Fe. Why weren't similar detrimental phases observed at 0.319 wt. %?
A2: The paper confirms that these phases were not observed, but does not speculate on the metallurgical reason. The finding implies that 0.319 wt. % is below the critical threshold for the formation of these specific, large, needle-like intermetallic phases in the AlSi7Mg0.3 alloy under the casting and T6 heat treatment conditions used in the study.
Q3: What was the standard limit for iron in AlSi7Mg0.3 according to the standards cited?
A3: According to Table 1, which references the ČSN EN 1706 standard, the maximum allowable iron content for AlSi7Mg0.3 is 0.19 wt. %. The table also lists a typical value of 0.15 wt. %. This highlights that the test sample, at 0.319 wt. % Fe, was significantly above the accepted standard, making the finding that it did not affect corrosion resistance all the more impactful.
Q4: Did the increased iron content affect the mechanical properties or machinability of the casting?
A4: The paper explicitly states, "No influence or change in the machining process was observed during the processing of the part." However, it does not provide quantitative data on mechanical properties like tensile strength or elongation. The focus of the study was strictly on corrosion resistance.
Q5: Could other elements in the alloy, such as Manganese (Mn), have played a role in mitigating the effect of the higher iron?
A5: The chemical composition in Tables 2 and 3 shows Mn levels of 0.054 wt. % and 0.063 wt. %. The paper's introduction mentions that Mn can have a positive effect by forming (MnFe)Al6, which helps remove iron during melting. While the paper does not draw a direct conclusion on this point, it is a known metallurgical principle that could have contributed to the favorable outcome.
Conclusion: Paving the Way for Higher Quality and Productivity
This study provides compelling evidence that challenges the blanket assumption that any increase in iron content above the standard limit is detrimental to AlSi7Mg0.3 corrosion resistance. For the specific conditions tested, an iron level of 0.319 wt. %—well above the 0.19 wt. % standard—showed no negative impact in a rigorous 480-hour salt spray test. This suggests a significant opportunity for foundries to leverage more cost-effective secondary aluminum alloys without compromising the performance of components in certain corrosive environments. By understanding the true thresholds of impurity effects, we can make smarter material choices that enhance both quality and productivity.
At CASTMAN, we are committed to applying the latest industry research to help our customers achieve higher productivity and quality. If the challenges discussed in this paper align with your operational goals, contact our engineering team to explore how these principles can be implemented in your components.
Copyright Information
- This content is a summary and analysis based on the paper "Analysis of the Increased Iron Content on the Corrosion Resistance of the AlSi7Mg0.3 Alloy Casting" by "Jaroslava Svobodova, Milan Lunak, Michal Lattner".
- Source: https://doi.org/10.21062/ujep/415.2019/a/1213-2489/MT/19/6/1041
This material is for informational purposes only. Unauthorized commercial use is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.