Paper Title Al-Mg-Si Alloy Casting: A Comparative Study on Microstructure and Corrosion Performance
This technical summary is based on the academic paper "EFFECTS OF SPIN AND DIE CASTING ON MICROSTRUCTURE AND CORROSION BEHAVIOUR OF Al-Mg-Si ALLOY" by Henry Kayode TALABI, Benjamin Omotayo ADEWUYI, and Oladayo OLANIRAN, published in ACTA TEHNICA CORVINIENSIS – Bulletin of Engineering (2015).
Keywords
- Primary Keyword: Al-Mg-Si Alloy Casting
- Secondary Keywords: Die Casting Corrosion, Spin Casting Microstructure, Sand Casting, Corrosion Resistance, Aluminium Alloy Performance
Executive Summary
- The Challenge: Selecting the optimal casting method for Al-Mg-Si alloys is critical for ensuring desired corrosion resistance in demanding automotive, aerospace, and industrial applications.
- The Method: An Al-Mg-Si alloy was produced using spin, die, and sand casting methods, and its corrosion behavior was evaluated in various concentrations of H₂SO₄ (acid) and NaCl (saline) environments.
- The Key Breakthrough: Die casting demonstrated significantly better corrosion resistance in acidic (H₂SO₄) environments compared to spin and sand casting, while all three methods performed well in saline (NaCl) environments.
- The Bottom Line: For Al-Mg-Si alloy components intended for service in acidic conditions, die casting is the superior manufacturing method for maximizing corrosion resistance and service life.
The Challenge: Why This Research Matters for HPDC Professionals
Aluminum alloys, particularly the Al-Mg-Si series, are foundational materials in the aerospace and automotive industries due to their low density, high specific strength, and inherent corrosion resistance. However, the final performance of a component is not determined by the alloy alone; the manufacturing process plays a pivotal role. The properties achieved through one casting method may not be identical to those from another, even with the same alloy. This research addresses a critical question for engineers and designers: how do common casting methods—specifically spin, sand, and die casting—affect the microstructure and, consequently, the corrosion behavior of an Al-Mg-Si alloy? Understanding this relationship is essential for selecting the right process to guarantee component reliability and longevity, especially in corrosive service environments.
The Approach: Unpacking the Methodology
The researchers conducted a comparative study using a custom-produced Al-Mg-Si alloy.
- Materials: The alloy was created from aluminium scrap, magnesium, and silicon. The final chemical composition after casting was confirmed via a spectrometric analyzer to be 98.68% Al, 0.55% Mg, and 0.40% Si (see Table 1 in the paper for full composition).
- Casting Methods: Three distinct casting processes were used to produce test samples:
- Spin Casting: Molten metal was poured into a temporary sand mold held in a spinning chamber, using centrifugal force.
- Sand Casting: A conventional two-piece sand mold was created by packing sand around a pattern.
- Die Casting: A reusable die mold was prepared from cast iron.
- Analysis and Testing: The cast products were evaluated for porosity based on density measurements. Microstructural analysis was performed to observe the dispersion of alloying elements. For corrosion testing, samples were polished, degreased, and immersed in acidic (0.1M, 0.3M, and 0.5M H₂SO₄) and saline (0.1M, 0.3M, and 0.5M NaCl) solutions for 60 days. Corrosion behavior was quantified by measuring mass loss and calculating the corrosion rate over time.
The Breakthrough: Key Findings & Data
The study revealed clear performance differences between the casting methods, particularly regarding microstructure and corrosion resistance in acidic environments.
Finding 1: Spin Casting Achieves Superior Microstructure and Lower Porosity
The investigation into the physical properties of the cast samples showed that the spin casting method produced the most solid part.
- Porosity: As shown in Table 1, spin casting resulted in a porosity of only 0.37%. In contrast, both sand casting and die casting produced samples with a higher porosity of 1.11%.
- Microstructure: Micrographs revealed that in the spin-cast sample, "magnesium and silicon were better dispersed in aluminium matrix" (Figures 1-3). This indicates a more homogenous material structure compared to the other methods.
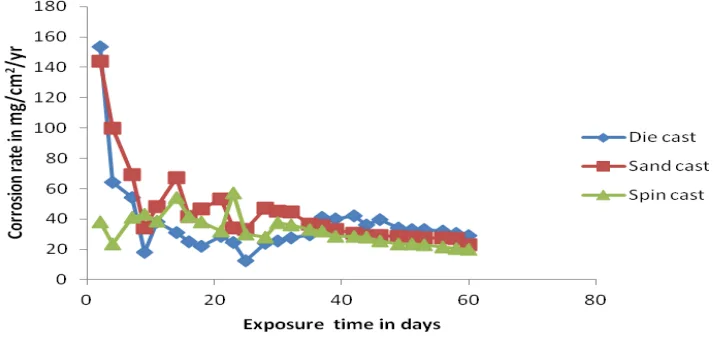
Finding 2: Die Casting Delivers Superior Corrosion Resistance in Acidic Environments
Despite having higher porosity, the die-cast samples consistently outperformed the others in the sulfuric acid (H₂SO₄) tests across all concentrations.
- In 0.1M H₂SO₄: Figure 4.1 shows that while sand and die casting had an initial corrosion peak at day two, the corrosion rate for the die-cast sample remained significantly lower than the others over the 60-day period.
- In 0.3M H₂SO₄: As seen in Figure 4.3, "the die casting has the lowest corrosion rate" throughout the test, while sand casting exhibited the highest.
- In 0.5M H₂SO₄: The trend continued in the most concentrated acid. Figure 4.5 clearly illustrates that "die casting has the lowest corrosion rate while the sand casting has the highest corrosion rate." The die-cast sample’s corrosion rate dropped significantly after the second day and remained low, suggesting the formation of a stable passive film.
Practical Implications for R&D and Operations
- For Process Engineers: This study suggests that for components exposed to acidic industrial fluids or environments, specifying die casting may contribute to significantly longer service life and reduced material degradation. While spin casting produces a denser part, die casting offers superior protection in these specific corrosive conditions.
- For Quality Control Teams: The data in Figures 4.1, 4.3, and 4.5 of the paper illustrates the profound effect of the casting method on corrosion rate. This could inform new quality inspection criteria for parts where corrosion resistance is a critical-to-quality (CTQ) characteristic.
- For Design Engineers: The findings indicate that the choice of manufacturing process is as critical as material selection for parts designed for corrosive environments. The superior performance of die casting in H₂SO₄ suggests this method should be prioritized during the design phase for relevant applications.
Paper Details
EFFECTS OF SPIN AND DIE CASTING ON MICROSTRUCTURE AND CORROSION BEHAVIOUR OF Al-Mg-Si ALLOY
1. Overview:
- Title: EFFECTS OF SPIN AND DIE CASTING ON MICROSTRUCTURE AND CORROSION BEHAVIOUR OF Al-Mg-Si ALLOY
- Author: Henry Kayode TALABI, Benjamin Omotayo ADEWUYI, Oladayo OLANIRAN
- Year of publication: 2015
- Journal/academic society of publication: ACTA TEHNICA CORVINIENSIS – Bulletin of Engineering, Tome VIII, Fascicule 4
- Keywords: Al-Mg-Si alloy, spin casting, sand casting, corrosion, spectrometric analyser
2. Abstract:
The microstructure and corrosion behavior of Al-Mg-Si alloy using spin, die and sand casting was investigated. The materials used were aluminium scrap, magnesium and silicon, they were all subjected to chemical analysis using spectrometric analyser. Charge calculation to determine the amount needed to be charged into the furnace was properly worked out and charged into the crucible furnace from which as-cast aluminium was obtained. Density measurements were used as a basis of evaluating the percentage porosity of the cast products; the corrosion behavior of the cast produced in acid 0.1M, 0.3M and 0.5M H₂SO₄ and saline 0.1M, 0.3 and 0.5M NaCl environment were investigated using corrosion rate, mass loss. From the results it was observed that magnesium and silicon were better dispersed in aluminium matrix of the spin casting. However, during the corrosion test in H₂SO₄, die casting exhibited best corrosion resistance followed by spin and sand casting. The spin, sand and die casting all exhibited good corrosion resistance in NaCl.
3. Introduction:
Casting is a fundamental manufacturing process where molten metal is poured into a mold to create a desired shape. Different techniques like sand, die, and spin casting are commonly used. Aluminium alloys are widely employed in aerospace and automobile industries for their low density, high specific strength, and corrosion resistance. The final properties of a cast aluminium component depend on three primary factors: the casting alloy, the melting process, and the casting method. This study was initiated because the properties obtained from one combination of these factors may not be identical to those achieved with the same alloy in a different casting facility, highlighting the need to understand how different casting methods impact performance.
4. Summary of the study:
Background of the research topic:
The performance of Al-Mg-Si alloys in structural components is highly dependent on the manufacturing process. While the alloy's composition provides baseline properties, the casting method influences microstructure, porosity, and ultimately, real-world durability, including corrosion resistance.
Status of previous research:
Previous research has established the benefits of aluminium alloys and various casting techniques. It has been noted that die casting is a versatile process for mass production [2], and spin casting can produce fine-grained structures [3]. Research has also highlighted the use of Al-alloys in aviation due to their properties [5, 6]. However, a direct comparative study of how spin, sand, and die casting specifically affect the corrosion behavior of an Al-Mg-Si alloy in both acidic and saline environments was needed.
Purpose of the study:
The purpose of this investigation was to determine the effects of three different casting methods—spin, die, and sand casting—on the microstructure and corrosion behavior of an Al-Mg-Si alloy.
Core study:
The core of the study involved producing Al-Mg-Si alloy samples using the three casting methods and subjecting them to extensive corrosion testing. The samples were immersed in H₂SO₄ and NaCl solutions of varying concentrations for 60 days. Researchers monitored mass loss and corrosion rates at regular intervals and analyzed the microstructure of each cast type to correlate the physical structure with the corrosion performance.
5. Research Methodology
Research Design:
The study employed a comparative experimental design. An Al-Mg-Si alloy was prepared and cast using three different methods. The resulting samples were then subjected to identical corrosion tests to allow for a direct comparison of their performance.
Data Collection and Analysis Methods:
- Material Analysis: The chemical composition of the base materials was determined using a spectrometric analyzer.
- Density and Porosity: Experimental densities were measured using a high-precision electronic weighing balance. Percentage porosity was calculated by comparing the experimental density to the theoretical density.
- Corrosion Testing: Samples were immersed in H₂SO₄ and NaCl solutions. Mass loss was measured every two days for 60 days using a digital analytical weighing machine. Corrosion rate was evaluated from the weight loss measurement following ASTM standard practice.
- Microstructural Analysis: Micrographs of the cast samples were taken to observe the grain structure and dispersion of alloying elements.
Research Topics and Scope:
The research focused on an Al-Mg-Si alloy. The scope included the fabrication of the alloy, casting via spin, sand, and die methods, and a detailed investigation of the resulting microstructure, density, and corrosion behavior in acidic (H₂SO₄) and marine (NaCl) environments.
6. Key Results:
Key Results:
- Spin casting produced the sample with the lowest porosity (0.37%) compared to sand and die casting (1.11%).
- The microstructure of the spin casting revealed that magnesium and silicon were more effectively dispersed within the aluminium matrix.
- In all concentrations of H₂SO₄ (acidic environment), die casting exhibited the best corrosion resistance, followed by spin and then sand casting.
- In all concentrations of NaCl (saline environment), all three casting methods (spin, sand, and die) exhibited good corrosion resistance.
Figure Name List:
- Figure 1. Micrograph of Spin Casting (x400)
- Figure 2. Micrograph of Sand Casting (x400)
- Figure 3. Micrograph of Die Casting (x400)
- Figure 4.1. Variation of corrosion rate of cast products in 0.1M H₂SO₄
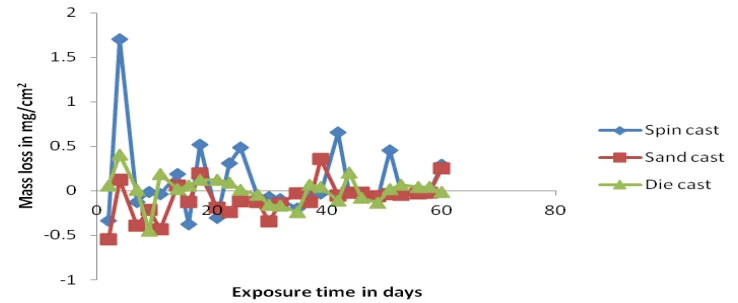
- Figure 4.2- Variation of mass loss against exposure time of cast products in 0.1M H₂SO₄
- Figure 4.3. Variation of corrosion rate of cast products in 0.3M H₂SO₄
- Figure 4.4. Variation of mass loss against exposure time of cast products in 0.3M H₂SO₄
- Figure 4.5. Variation of corrosion rate of cast products in 0.5M H₂SO₄
- Figure 4.6. Variation of mass loss against exposure time of cast products in 0.5 H₂SO₄
- Figure 4.7. Variation of corrosion rate of cast products in 0.1M NaCl
- Figure 4.8. Variation mass of loss against exposure time of cast products in 0.1M NaCl
- Figure 4.9. Variation of corrosion rate of cast products in 0.3M NaCl
- Figure 4.10. Variation of mass loss against exposure time of cast products in 0.3 NaCl
- Figure 4.11. Variation of corrosion rate of cast products in 0.5M NaCl
- Figure 4.12. Variation of mass loss against exposure time of cast product in 0.5 NaCl
7. Conclusion:
Based on the strength of the results presented, the following conclusions were drawn:
- The microstructure of the spin casting revealed that magnesium and silicon were well dispersed in the aluminium matrix as compared to sand and die casting.
- The die casting products exhibit better corrosion resistance in 0.1M, 0.3M, and 0.5M H₂SO₄ as compared with spin and die casting.
- The products produced from spin, sand and sand [sic] exhibit a good corrosion resistance in 0.1M, 0.3M, and 0.5M NaCl.
8. References:
- [1.] Callister, W.D.; Fundamentals Materials Science and Engineering, Ranjbaran, Wiley and Sons Inc. USA, Pp. 364-578, 2010
- [2.] Adewuyi, B.O.; Omotoyinbo, J.A.; Effect of Cooling Media on the Mechanical Properties and Microstructure of Sand and Die casting Aluminium Alloys. Journal of Science and Technology, Volume 28, Pp. 97-100, 2008.
- [3.] Polmear, I.J.; Production of Aluminium. Light Alloys from Traditional Alloys to Nanocrystals. Oxford Elsevier/ Butterworth-Hememann, Pp. 15-16, 2006.
- [4.] Yazdiam, N.; Kazimzadeh, F.; Tovoosi, M.; Microstructural Evolution of Nanostructure 7075 Aluminium Alloy during Isothermal Annealing. Journal of Alloys and Compounds, 493 Pp. 137-141, 2010.
- [5.] Prabhu, C.; Suryanarayana, C.; An, L.; Vaidyanathan, R.; Synthesis and Characterization of High Volume Fraction Al-A1203 Nanocomposite powders by high energy milling. Journal of Material Science Engineering A, Volume 425, No. 1-2, Pp. 192-200, 2006.
- [6.] Torralba, J.M.; Velasco, F.; Costa, C.E.; Vergara, I.; Caceres, D.; Mechanical behaviour of the Interphase between Matrix and Reinforcement of Al 2014 Matrix Composites Reinforced with (Ni3Al)p, 2002.
- [7.] Hizombor, M.; Mirbagheri, S.M.H.; Abdideh, R.; Casting of A356/TiB2p Composite Based on the TiB2p/CMC/PPS Mortar Roznov pod Radhostem, Czech Republic, Volume 5, Pp. 18-20, 2010.
- [8.] Hashim, J.; Looney, L.; Hashim, M. S. J.; Metal Matrix Composites: Production by Stir Casting Method, Mat. Proc. Tech Volume 92, Pp. 1-7, 1999.
- [9.] Wang B.B.; Wang Z.Y.; Han W. and Ke W. ; Atmospheric Corrosion of Aluminium Alloy 2024-T3 Exposed to Salt Environment in Western China, Journal of Corrosion Science, Volume 59, Pp. 63-70, 2012.
- [10.] Liang C.F., Hou W.T.; Twelve year Atmospheric Exposture of Stainless Steels in China, in; H.E. Townsent (Ed.). Outdoor Atmospheric Corrosion, ASTM STP 1421, American Society of Testing and Materials, Philadelphia, Pp. 358-367, 2002.
- [11.] Wang Z.Y., Li Q.X., Han W., Yu G.C., Han E. H.; Corrosion Behaviour of 316L Stainless Steel Exposed to Qin ghai Salt Lake Atmosphere, 5th Chinese Society for Corrosion and Protection, P. 115, 2009.
- [12.] Ekuma C.E., Idenyi N.E., Neife S.I.; Comparative Analysis of the Corrosion Susceptibility of Cast Al-Mn Alloys in Acidic Environment. Res. Journal of Environmental Service, 1Volume 4, P. 185, 2007.
- [13.] Oguzie E.; Corrosion Inhibition of Aluminium in Acidic and Alkaline Media Sansevieria trifaciata Extract . Journal of Corrosion Science, Volume 49, Pp. 1527-1539, 2007.
Expert Q&A: Your Top Questions Answered
Q1: What was the specific chemical composition of the Al-Mg-Si alloy used in the study?
A1: As detailed in Table 1 on the first page, the final chemical composition of the as-cast alloy was 0.40% Si, 0.24% Fe, 0.03% Cu, 0.04% Mn, 0.55% Mg, 0.03% Zn, 0.01% Cr, 0.02% Ti, and 98.68% Al. This composition was verified using a spectrometric analyzer after the materials were melted and cast.
Q2: The study mentions porosity. Which casting method yielded the most dense product?
A2: According to the density measurements presented in Table 1 on page 2, spin casting produced the product with the lowest porosity at 0.37%. Both sand casting and die casting resulted in a higher, identical porosity of 1.11%. This suggests the centrifugal forces in spin casting are effective at creating a more solid part.
Q3: Can you elaborate on the microstructural differences observed between the casting methods?
A3: The study concluded that "magnesium and silicon were better dispersed in aluminium matrix of the spin casting." A visual inspection of Figures 1, 2, and 3 supports this, showing a more uniform structure in the spin-cast sample. This improved dispersion is a key microstructural advantage of the spin casting process noted in this research.
Q4: Why did the die-cast sample perform best in the H₂SO₄ environment despite having higher porosity than the spin-cast sample?
A4: The paper does not provide a direct explanation for this specific outcome. However, the results consistently show that the die-cast sample had the lowest corrosion rate in all H₂SO₄ concentrations (Figures 4.1, 4.3, 4.5). This superior performance could be related to the nature of the surface finish, the rate of solidification affecting the passive layer formation, or other factors not detailed in the paper, but the empirical data clearly establishes die casting as the most resistant method in these acidic conditions.
Q5: The mass loss graphs for NaCl (e.g., Figure 4.8) show negative values. What does this indicate?
A5: The paper notes that in the NaCl environment, sand casting showed a "higher in weight gain." A negative mass loss indicates a net weight gain in the sample. This is typically due to the formation of a passive oxide or corrosion product layer on the surface that weighs more than the base metal that was lost to corrosion during the same period.
Q6: How was the corrosion rate determined over the 60-day test?
A6: The corrosion rate was evaluated from weight loss measurements, which were monitored at two-day intervals. According to the paper, the mass loss (in mg/cm²) for each sample was calculated by dividing the measured weight loss by its total surface area. This procedure is in accordance with ASTM standard practice and provides a standardized method for comparing the corrosion behavior of the different samples over time.
Conclusion: Paving the Way for Higher Quality and Productivity
This research provides valuable, data-driven insights for any engineer working with Al-Mg-Si alloy casting. The core takeaway is that the manufacturing process has a direct and significant impact on a component's ultimate corrosion resistance. While spin casting can produce a denser part with a more homogenous microstructure, die casting is the clear choice for applications requiring durability in acidic environments. This study underscores the importance of aligning the casting method with the component's intended service environment to maximize performance and reliability.
At CASTMAN, we are committed to applying the latest industry research to help our customers achieve higher productivity and quality. If the challenges discussed in this paper align with your operational goals, contact our engineering team to explore how these principles can be implemented in your components.
Copyright Information
- This content is a summary and analysis based on the paper "EFFECTS OF SPIN AND DIE CASTING ON MICROSTRUCTURE AND CORROSION BEHAVIOUR OF Al-Mg-Si ALLOY" by "Henry Kayode TALABI, Benjamin Omotayo ADEWUYI, Oladayo OLANIRAN".
- Source: ACTA TEHNICA CORVINIENSIS – Bulletin of Engineering, Tome VIII [2015] Fascicule 4 [October – December], ISSN: 2067-3809
This material is for informational purposes only. Unauthorized commercial use is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.