The content of this introduction paper is based on the article "DIE SOLDERING IN ALUMINUM DIE CASTING" published by "Oak Ridge National Laboratory".
1. Overview:
- Title: DIE SOLDERING IN ALUMINUM DIE CASTING
- Author: Q. Han, E. A. Kenik, and S. Viswanathan
- Year of publication: Not specified in provided text (Referenced works up to 1999; Contract suggests 1996 or later)
- Journal/academic society of publication: Oak Ridge National Laboratory (Metals and Ceramics Division) - Manuscript/Report
- Keywords: Die casting, aluminum alloys, die soldering, die sticking, intermetallics, Fe-Al system, critical temperature, microstructure, dipping test, dip-coating test.
2. Abstract:
Two types of tests, "dipping" tests and "dip-coating" tests, were carried out on small steel cylinders using pure aluminum and 380 alloy to investigate the mechanism of die soldering during aluminum die casting. Optical and scanning electron microscopy were used to study the morphology and composition of the phases formed during soldering. A soldering mechanism is postulated based on experimental observations. A soldering critical temperature is postulated at which iron begins to react with aluminum to form an aluminum-rich liquid phase and solid intermetallic compounds. When the temperature at the die surface is higher than this critical temperature, the aluminum-rich phase is liquid and joins the die with the casting during the subsequent solidification. The paper discusses the mechanism of soldering for the case of pure aluminum and 380 alloy casting in a steel mold, the factors that promote soldering, and the strength of the bond formed when soldering occurs.
3. Introduction:
Soldering, or die sticking, in die casting occurs when molten aluminum adheres ("welds") to the die surface, leading to die damage and reduced casting surface quality. Literature identifies two primary types: high-temperature soldering driven by chemical/metallurgical reactions between the molten alloy and the die, and low-temperature soldering due to mechanical interaction. This study focuses exclusively on the high-temperature chemical/metallurgical reaction type. This form of soldering is often associated with the "washout" of protective films (coatings or lubricants) on the die surface, allowing direct contact between molten aluminum and the die steel. Subsequent dissolution of iron into the melt and diffusion of aluminum and other elements into the die result in the formation of an intermetallic layer at the die surface. Under specific conditions, an aluminum-rich soldering layer may form over this intermetallic layer. While significant research exists on the nature of these intermetallics, the precise conditions initiating soldering are less understood. This study differentiates between the mere formation of intermetallics and the occurrence of soldering, focusing on the initiation phase. It aims to address: 1. The temperatures at which soldering occurs; 2. Whether intermetallic formation signifies soldering; 3. The mechanism by which an aluminum alloy casting solders (joins) to the die; 4. The factors determining the strength of the resulting bond.
4. Summary of the study:
Background of the research topic:
Die soldering (die sticking) represents a critical challenge in aluminum die casting, stemming from the adhesion of the cast aluminum alloy to the steel die surface. This phenomenon leads to operational inefficiencies, increased tooling costs due to die damage, and compromised quality of the cast components. The study specifically addresses soldering resulting from high-temperature chemical/metallurgical interactions.
Status of previous research:
Prior research acknowledged the link between soldering, the breakdown of protective die surface films ("washout"), and the subsequent formation of iron-aluminum intermetallic compounds at the die-alloy interface. However, a comprehensive understanding of the specific conditions (e.g., temperature, composition) required to initiate soldering, as distinct from simple intermetallic growth, was lacking. Existing literature (references 3-7) detailed the nature of intermetallics but offered limited insight into soldering initiation.
Purpose of the study:
The primary objective was to elucidate the fundamental mechanism of die soldering initiation in aluminum die casting involving steel dies. This involved:
- Investigating the critical temperature conditions required for soldering to occur with pure aluminum and 380 alloy.
- Determining if the presence of intermetallic phases alone is sufficient evidence of soldering.
- Postulating the mechanism by which the aluminum casting metallurgically bonds (joins) to the steel die during soldering.
- Identifying factors that influence the strength of the solder bond.
Core study:
The core of the study involved controlled laboratory experiments simulating aspects of the die-casting environment. Small mild steel cylinders were subjected to "dipping" and "dip-coating" tests using molten pure aluminum and 380 aluminum alloy. Sample surface temperatures were carefully monitored and controlled relative to the alloys' melting/liquidus points. The interfaces formed between the steel and aluminum were subsequently analyzed using optical and scanning electron microscopy (SEM) combined with compositional analysis to characterize the morphology and chemical makeup of the reaction products (intermetallics and other phases). Based on these observations, a mechanism for soldering initiation was proposed.
5. Research Methodology
Research Design:
The study employed an experimental approach using immersion tests. Two main procedures were utilized:
- Dipping tests: Steel samples were immersed in molten aluminum or 380 alloy held at a temperature 30°C higher than the target sample surface temperature. The sample was moved in and out of the melt to maintain the desired surface temperature (±3°C), monitored by a welded thermocouple. Tests were conducted at surface temperatures both above and below the alloy's melting point (pure Al) or liquidus temperature (380 alloy).
- Dip-coating tests: For tests below the alloy's liquidus/melting point, cold steel samples were dipped briefly to acquire a coating of the alloy, then moved to a furnace set at the desired test temperature and held for specific durations.
Data Collection and Analysis Methods:
- Temperature Measurement: Type K thermocouples were welded to the surface of the mild steel cylinder samples (12 mm diameter, 25 mm length) to monitor and control the interface temperature during testing.
- Microstructural Analysis: After testing and air cooling, samples were sectioned, polished, and examined using optical microscopy and a Phillips XL30/FEG scanning electron microscope (SEM).
- Compositional Analysis: SEM equipped with energy-dispersive X-ray spectroscopy (EDS) or similar was used to determine composition profiles across the interface between the steel substrate, intermetallic layers, and the aluminum alloy. The reported accuracy for compositional analysis was approximately 3%.
Research Topics and Scope:
The research focused on the interaction between mild steel and two aluminum materials: pure aluminum and 380 aluminum alloy. The scope was limited to understanding the initiation of soldering caused by chemical/metallurgical reactions at elevated temperatures. It explicitly excluded soldering mechanisms based on low-temperature mechanical interactions. The study investigated the influence of die (sample) surface temperature and contact time on the formation of intermetallic phases and the occurrence of soldering. The morphology and composition of phases formed at the interface were central to the investigation.
6. Key Results:
Key Results:
- Soldering was observed in pure aluminum tests when the steel sample surface temperature (TD) was ≥ 657°C. No soldering occurred below 643°C, even after extended holding times (20 h).
- For 380 alloy, soldering occurred at TD ≥ 568°C but not at ≤ 550°C.
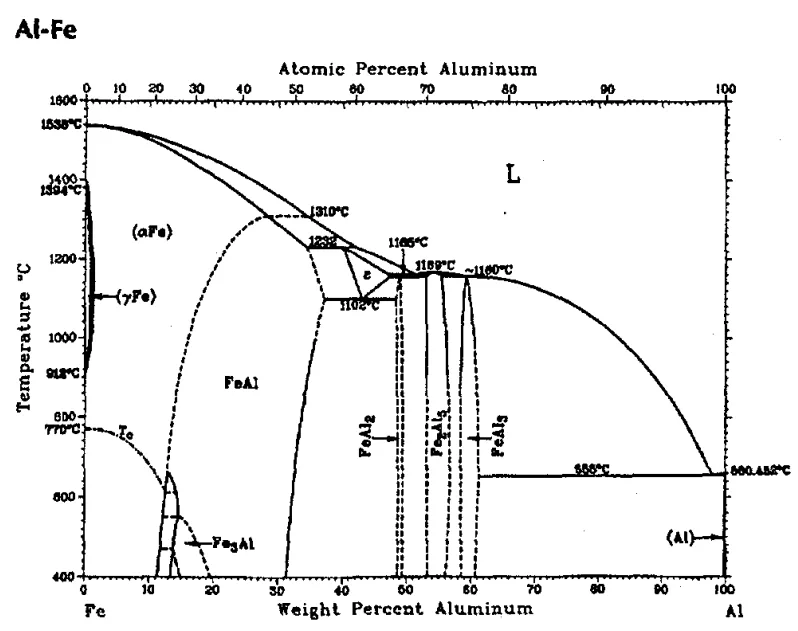
- A critical temperature (TC) for soldering initiation is proposed. For pure Al / steel, TC is correlated with the Fe-Al eutectic temperature (655°C). Soldering occurs if TD > TC and the local aluminum concentration at the die surface is high enough (> 61.3 wt% based on the Fe-Al phase diagram) to form an aluminum-rich liquid phase.
- For 380 alloy, TC appears to be below the liquidus (575°C) and potentially related to the dendrite coherency temperature (~560°C), although confirmation under actual die casting conditions is needed.
- The formation of intermetallics (e.g., FeAl3) on the die surface indicates a reaction occurred but does not necessarily mean soldering has occurred. Soldering was only observed when an interlocked structure consisting of both the intermetallic phase (FeAl3) and an aluminum-iron eutectic phase (aluminum + FeAl3) was present, indicating a metallurgical bond (Figure 2a, Figure 4).
- The presence of a gap between the aluminum and the steel, even with intermetallics present on the steel (Figure 2c), signifies the absence of soldering.
- The key factor for soldering (joining) is the formation of an aluminum-rich liquid phase at the interface (TD > TC), which exists in contact with and between the solid intermetallic phase. This liquid acts as a "glue".
- Upon cooling, this liquid solidifies, forming the eutectic component of the interlocked structure that bonds the casting to the die. The presence of pores within both eutectic and intermetallic regions (Figure 5) supports the existence of this liquid phase prior to solidification.
- The strength of the solder bond depends on the total area over which the bond forms and, within that area, the area fraction of the metallurgical bond derived from the solidified aluminum-rich liquid phase.
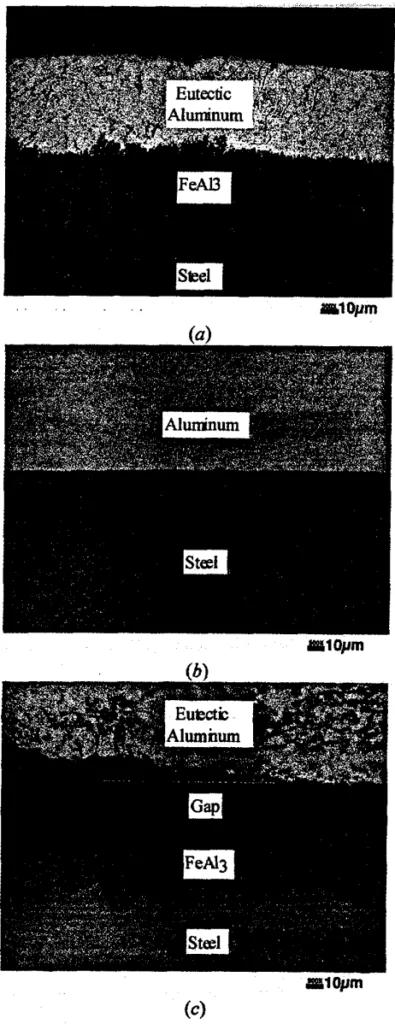
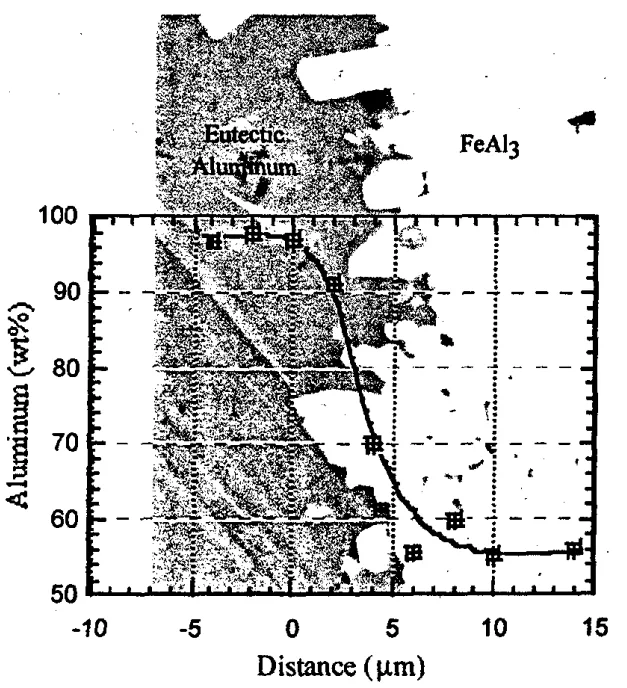

Figure Name List:
- Figure 1: Schematic illustration of the experimental setup for the dipping tests.
- Figure 2: Three typical microstructures observed at the die surface for three different cases: (a) soldering resulting in a metallurgical bond, (b) absence of reaction between steel and aluminum, and (c) intermetallic formation on the die in the absence of soldering.
- Figure 3: The aluminum-iron phase diagram (8).
- Figure 4: Composition profile across the aluminum/intermetallic interface in the soldered layer superimposed on a backscattered scanning electron image of the interface.
- Figure 5: An optical photomicrograph of the aluminum/ intermetallic interface indicating the presence of pores in both the aluminum and the intermetallic phases.
7. Conclusion:
This study investigated the mechanism of die soldering in aluminum die casting using dipping and dip-coating tests with pure Al and 380 alloy on steel samples. A soldering mechanism based on experimental observations was postulated:
- Soldering occurs when the die surface temperature (TD) exceeds a critical temperature (TC). For pure Al/steel, TC corresponds to the Fe-Al eutectic temperature (655°C). For 380 alloy, TC appears related to the dendrite coherency temperature (~560°C), but further validation is required.
- Above TC, provided there are no diffusion barriers (like oxides or lubricants), iron dissolves into the aluminum melt, and aluminum diffuses into the steel, leading to the formation of solid Fe-Al intermetallic phases (e.g., FeAl3).
- Crucially, if the aluminum concentration at the die surface becomes sufficiently high (e.g., > 61.3 wt% for pure Al), a small fraction of an aluminum-rich liquid phase forms in contact with and between the solid intermetallic phase.
- This liquid phase acts as the joining agent. Upon solidification, it forms an interlocked microstructure with the intermetallic phase, creating a strong metallurgical bond between the casting and the die.
- The mere presence of intermetallics does not constitute soldering; the formation of the intervening liquid phase is essential for bonding.
- The strength of the bond depends on the bonded area and the local volume fraction of the liquid phase formed prior to solidification, which is influenced by local temperature and composition.
These findings align with industrial observations that higher casting and die temperatures promote soldering. Factors increasing heat transfer to the die (e.g., high gate velocity, high intensification pressure) or alloys with high latent heat (e.g., 390-type alloys) are also likely to increase die surface temperature and thus promote soldering.
8. References:
- [1] Y. L. Chu, P. S. Cheng, and R. Shivpuri, "Soldering Phenomenon in Aluminum Die Casting: Possible Causes and Cures," Transactions (Rosemont, Illinois: North American Die Casting Association, 1993), 361-371.
- [2] D. Argo, R. J. Barnhurst, and W. Walkington, "NADCA Sponsored Research: The Causes of Soldering in Zinc Die Casting," Transactions (Rosemont, Illinois: North American Die Casting Association, 1997), 77-82.
- [3] V. G. Rivlin and G. V. Raynor, "Critical Evaluation of Constitution of Aluminum-Iron-Silicon System," Int. Met. Rev., 26 (1981), 133-152.
- [4] W. Kajoch and A. Fajkiel, "Testing the Soldering Tendencies of Aluminum Die Casting Alloys," Transactions (Rosemont, Illinois: North American Die Casting Association, 1991), 67-74.
- [5] R. W. Richards, R. D. Jones, P. D. Clements, and H. Clarke, "Metallurgy of Continuous Hot Dip Aluminizing," Int. Mat. Rev., 39 (1994), 191-212.
- [6] S. Shankar and D. Apelian, "Die Soldering - A Metallurgical Analysis of the Molten Aluminum/Die Interface Reactions," Transactions (Rosemont, Illinois: North American Die Casting Association, 1997), 243-251.
- [7] Y. L. Chu, S. Balasubramaniam, R. Rajan, and R. Shivpuri, "A Study of the Cast Alloy Die Surface Interactions in Aluminum Die Casting," Transactions (Rosemont, Illinois: North American Die Casting Association, 1997), 205-212.
- [8] H. Baker et al., eds., ASM Handbook, vol. 3, Alloy Phase Diagrams (Materials Park, OH: ASM International), 1992.
- [9] L. Arnberg, L. Bäckerud, and G. Chai, Solidification Characteristics of Aluminum Alloys, vol.3, Dendrite Coherency (American Foundrymen's Society, Inc., 1996), 115.
- [10] Z. W. Chen and M. Z. Jahedi, "Formation of Die/Casting Interaction Layer during High-Pressure Die Casting of Aluminum Alloys", Tooling 99, Melbourne, (March 1999), 165-169.
9. Copyright:
- This material is a summary of a paper by "Q. Han, E. A. Kenik, and S. Viswanathan". Based on "DIE SOLDERING IN ALUMINUM DIE CASTING".
- Source of the paper: [DOI URL not provided in the original document]
This material is summarized based on the above paper, and unauthorized use for commercial purposes is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.