This introduction paper is based on the paper "Mechanical Properties of Salt Core Comprised of Alkali Carbonate and Alkali Chloride" published by "J. JFS".
1. Overview:
- Title: Mechanical Properties of Salt Core Comprised of Alkali Carbonate and Alkali Chloride
- Author: Jun Yaokawa, Daisuke Miura, Koichi Anzai, Youji Yamada and Hiroshi Yoshii
- Year of publication: 2006
- Journal/academic society of publication: J. JFS (Journal of the Japan Foundry Engineering Society)
- Keywords: salt core, expendable core, carbonate, chloride, die casting, strength, microstructure
2. Abstract:
The strength of salt core comprised of NaCl-Na2CO3, KCI-K2CO3, KC1-NaCl and K2CO3-Na2CO3 binary salt systems was investigated in order to develop expendable core for high pressure die casting using 4-point bending test, Vickers hardness measurement, and SEM observation of solidification structures and fracture surfaces. Bending specimens were fabricated from 10 K superheated molten salts by the permanent mold method. The results of the bending test showed that the KCI-K2CO3 binary system offers quite high strength exceeding about 20 MPa. Moreover, especially high strength was obtained for the NaCl-Na2CO3 system whose strength value is higher than that of the KCI-K2CO3 system. Also, the Vickers hardness of the NaCl-Na2CO3 system is higher than that of KCI-K2CO3 system. In contrast to these binary systems, salt cores made of KC1-NaCl and K2CO3-Na2CO3 binary salt systems could not be strengthened by mixing salt, due to phase separation of the solid solution phase crystallized from molten salt.
In the solidification structures of NaCl-Na2CO3 system, dendritic or elliptic shaped primary phase and fine eutectic phase were found. As crack propagation is deflected by both the primary phase and fine eutectic phase, salt cores can be strengthened.
3. Introduction:
Die casting is a widely used manufacturing process for aluminum alloy castings due to its high productivity, excellent surface finish, and dimensional accuracy, enabling the production of thin-walled products. However, forming products with complex undercut features, such as closed-deck cylinder blocks, is challenging in die casting because it necessitates the use of expendable cores. A significant issue is the potential destruction of these cores by the high-velocity impact of molten metal during injection, preventing them from maintaining their shape. While strengthening the core can be a countermeasure, it often compromises collapsibility, making removal from the product difficult and labor-intensive. Therefore, there is a strong demand for expendable cores that balance both strength and ease of removal. This paper investigates the mechanical properties of salt cores made from mixtures of alkali carbonates and alkali chlorides as potential candidates for such applications.
4. Summary of the study:
Background of the research topic:
The primary challenge in die casting complex undercut shapes is the survivability and subsequent removal of expendable cores. Cores must withstand the harsh conditions of high-velocity molten metal injection yet be easily removed from the final casting. Existing core technologies often struggle to meet these conflicting requirements. Salt-based cores offer potential advantages, such as controlled melting points through mixing and the possibility of achieving high strength.
Status of previous research:
Previously developed expendable cores include sintered salt cores1), specially treated sand cores2,3), and metallic insert cores4). Each has its merits but also drawbacks related to limitations on injection speed, shape freedom, and cost. The authors' group previously proposed salt cores made by melt-forming, reinforced with ceramic materials like aluminum borate whiskers in alkali halides, achieving high strengths of 20-30 MPa5,6). However, these faced challenges such as the high melting point of alkali halides (around 1000 K), whisker agglomeration leading to reduced strength, and complex whisker recycling processes. Patents exist for low-melting point salt cores using mixed salts7) and unreinforced salt cores8). Trials of melt-forming salt cores for die casting have also been conducted9), but practical examples are scarce. Despite the potential of mixed salts for improved productivity, controlled liquidus temperatures, and high strength, fundamental research on these materials has been limited.
Purpose of the study:
This study aimed to investigate the strength, hardness, and microstructure of binary mixed salt systems—specifically combinations of potassium chloride (KCI), sodium chloride (NaCl), potassium carbonate (K2CO3), and sodium carbonate (Na2CO3)—to provide fundamental data for the development of salt cores for die casting.
Core study:
The research focused on the mechanical properties and microstructures of four binary salt systems: NaCl-Na2CO3, KCI-K2CO3, KCI-NaCl, and K2CO3-Na2CO3.
5. Research Methodology
Research Design:
Test specimens were fabricated using 99.5% purity KCI, NaCl, K2CO3, and Na2CO3. Binary mixtures of these salts (180g batches) with compositions as shown in Table 1 were melted in an alumina Tammann crucible in an electric furnace under an air atmosphere. The molten salts, superheated by 10 K, were poured into a JIS-SCM440 steel permanent mold preheated to 373 K. The casting design for bending test specimens is shown in Fig. 1. Specimens were removed from the mold 60 seconds after pouring and air-cooled.
Data Collection and Analysis Methods:
- Bending Strength: Determined by a 4-point bending test with a lower support span of 50 mm, an upper loading span of 10 mm, 4 mm diameter loading pins, and a test speed of 1.6 × 10⁻² mm·s⁻¹ (1 mm·min⁻¹). Bending strength (σ) was calculated using equation (1), assuming a rectangular cross-section of H=20 mm and B=18 mm for a conservative estimate.
σ = 3LP / BH² (1) - Vickers Hardness: Measured on polished cross-sections near the fracture surface using a micro-Vickers hardness tester.
- Microstructure Analysis: Solidification structures were observed using a Scanning Electron Microscope (SEM) after dry polishing specimens with #4000 grit paper, ultrasonic cleaning, and carbon coating. Energy Dispersive X-ray spectroscopy (EDX) was used for local chemical analysis.
- Phase Identification: X-ray Diffraction (XRD) was used to identify phases present in the solidified structures near the fracture surface.
- Fracture Surface Analysis: Fracture surfaces were carbon-coated and observed by SEM with EDX analysis to investigate fracture and strengthening mechanisms.
Research Topics and Scope:
The study encompassed the evaluation of bending strength and Vickers hardness of the four binary salt systems across various compositions. It also included detailed microstructural characterization, phase identification, and fractography to understand the relationship between composition, microstructure, and mechanical properties, particularly focusing on strengthening mechanisms.
6. Key Results:
Key Results:
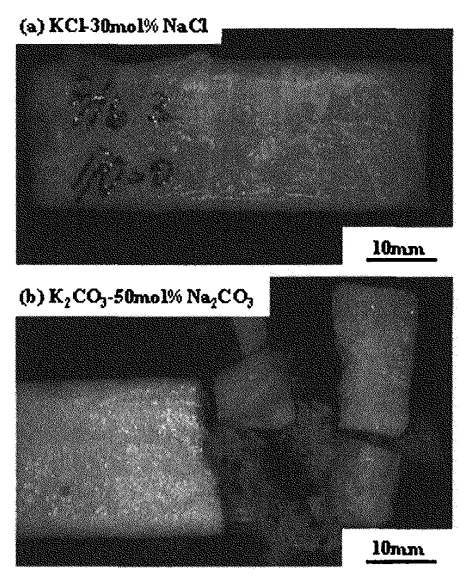
- Casting Defects: Specimens of KCI-30mol%NaCl exhibited cracks and surface unevenness (Fig. 2a). K2CO3-50mol%Na2CO3 specimens were very brittle (Fig. 2b). Internal shrinkage and cracks were common, for example in KCI-80mol%K2CO3 specimens (Fig. 3).
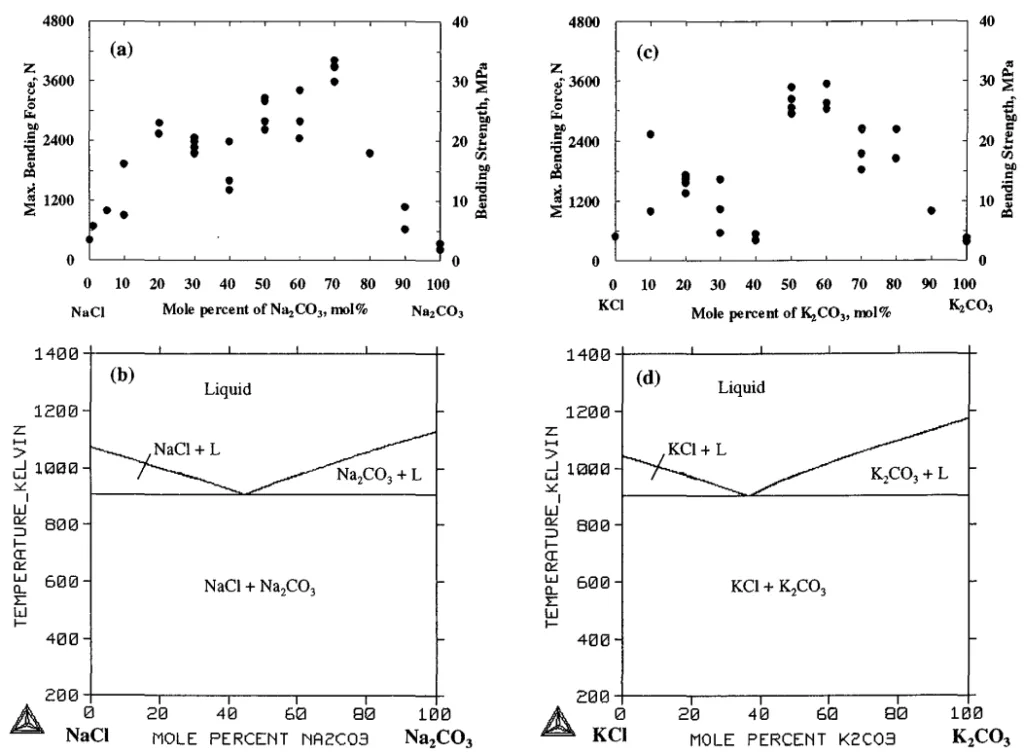
- Strength of NaCl-Na2CO3 and KCI-K2CO3 Systems (Fig. 4):
- Pure NaCl and Na2CO3 showed low strength (3-5 MPa). However, mixing significantly increased strength. NaCl-70mol%Na2CO3 achieved a high strength of 29-34 MPa.
- The KCI-K2CO3 system also showed high strength, with KCI-50~60mol%K2CO3 compositions exceeding 25 MPa. These strengths are comparable to those of whisker-reinforced salt cores and significantly higher than conventional sand cores (approx. 6 MPa).
- Strength of KCI-NaCl and K2CO3-Na2CO3 Systems (Fig. 5):
- Both KCI-NaCl and K2CO3-Na2CO3 binary systems exhibited very low strength, with some KCI-NaCl samples having near-zero strength. This was attributed to the formation of a complete solid solution (KxNa1-xCl or KxNa2-xCO3) from the melt, which subsequently undergoes phase separation upon cooling to room temperature. This phase separation, coupled with lattice misfit, leads to extreme brittleness and cracking (as seen in Fig. 2a, 2b).
- Vickers Hardness (Fig. 6):
- The NaCl-Na2CO3 system generally exhibited higher Vickers hardness than the KCI-K2CO3 system across all compositions.
- Pure Na2CO3 was harder than pure NaCl, KCI, or K2CO3.
- The overall trend in hardness correlated with the observed bending strength, with harder compositions generally being stronger.
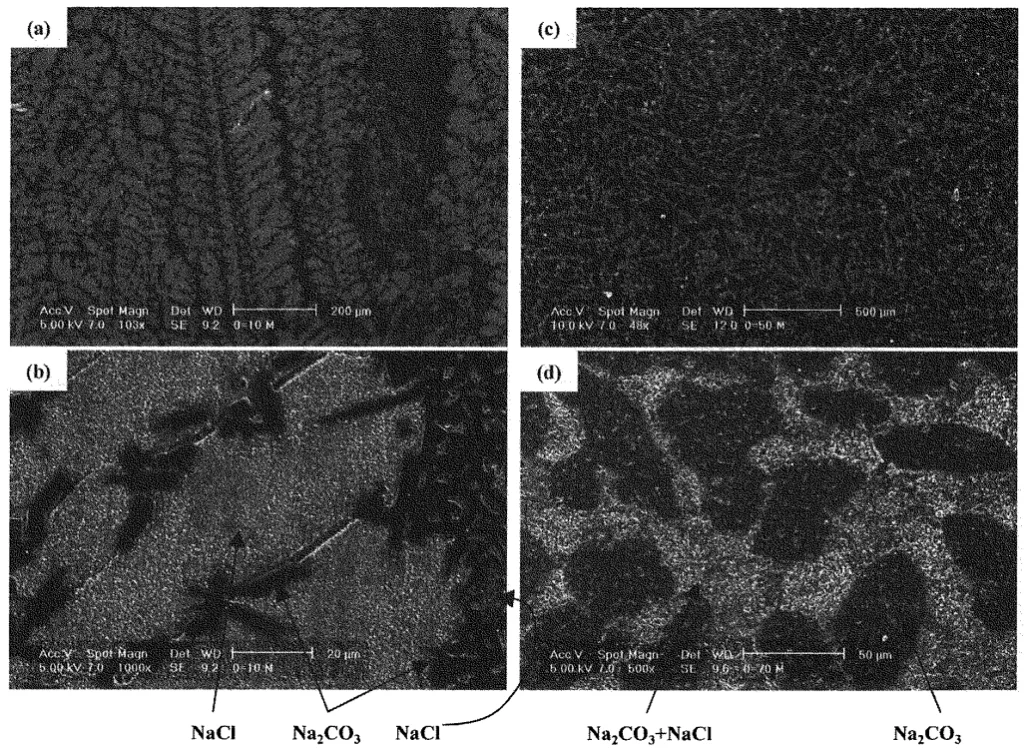
- Solidification Structure of NaCl-Na2CO3 System (Fig. 7, Fig. 8):
- For NaCl-10mol%Na2CO3, the microstructure consisted of primary NaCl dendrites with an interdendritic eutectic of NaCl and Na2CO3 (Fig. 7a, 7b).
- For NaCl-70mol%Na2CO3, the microstructure showed primary, elliptically shaped Na2CO3 surrounded by a eutectic of Na2CO3 and NaCl (Fig. 7c, 7d).
- SEM-EDX and XRD analysis (Fig. 8 for NaCl-70mol%Na2CO3) confirmed that the NaCl and Na2CO3 phases were nearly pure, with negligible solid solubility, consistent with the phase diagram (Fig. 4b).
- Fracture Surface Analysis of NaCl-Na2CO3 System (Fig. 9):
- In NaCl-10mol%Na2CO3, the fracture surface showed cleavage facets of primary NaCl (likely {001} planes) and fracture through the eutectic regions (Fig. 9a).
- In NaCl-70mol%Na2CO3, the fracture surface revealed fracture of the primary Na2CO3 phase and the surrounding eutectic (Fig. 9b).
- The presence of fine primary phases (approx. 50 µm) dispersed within the eutectic matrix is believed to strengthen the salt core by deflecting propagating cracks (crack deflection mechanism). The fine eutectic structure itself also contributes to strength by mitigating stress concentrations.
Figure Name List:
- Fig. 1 Casting design of bending test specimens.
- Fig. 2 Photographs of specimens at room temperature. (a) A lot of cracks are found in KCI-30 mol%NaCl specimen. (b) K2CO3-50 mol%Na2CO3 specimen shows very brittle behavior.
- Fig. 3 Cross section of KCI-80 mol%K2CO3 specimen.
- Fig. 4 Strength and phase diagrams of NaCl-Na2CO3 and KCI-K2CO3 binary systems10).
- Fig. 5 Strength and phase diagrams of KCI-NaCl and K2CO3-Na2CO3 binary systems10).
- Fig. 6 Vickers hardness of NaCl-Na2CO3 and KCI-K2CO3 binary systems. Solid and dotted lines connect average hardness value of each composition, respectively.
- Fig. 7 SEM secondary electron images of solidified structure. (a) and (b): NaCl - 10mol% Na2CO3. (c) and (d): NaCl -70mol% Na2CO3.
- Fig. 8 X-ray diffraction pattern of NaCl-70 mol% Na2CO3.
- Fig. 9 SEM images of broken surface. (a): NaCl-10 mol% Na2CO3. (b): NaCl-70 mol% Na2CO3.
7. Conclusion:
(1) Evaluation of the strength of four binary salt systems (NaCl-Na2CO3, KCI-K2CO3, KCI-NaCl, K2CO3-Na2CO3) revealed that high strengths exceeding 20 MPa were achieved in NaCl-Na2CO3 and KCI-K2CO3 systems. The NaCl-Na2CO3 system showed higher strength overall than the KCI-K2CO3 system. Notably, NaCl-70mol%Na2CO3 exhibited a very high strength of approximately 30 MPa, about five times that of conventional sand cores. Conversely, KCI-NaCl and K2CO3-Na2CO3 mixed salts were extremely brittle and had low strength, attributed to phase separation of the crystallized solid solution, leading to embrittlement and cracking.
(2) Vickers hardness measurements showed that the NaCl-Na2CO3 system was harder than the KCI-K2CO3 system over the entire composition range.
(3) The solidification microstructures of NaCl-Na2CO3 mixed salts were consistent with predictions from the phase diagram. On the NaCl-rich side of the eutectic, primary NaCl and a eutectic structure were observed. On the Na2CO3-rich side, primary Na2CO3 and a eutectic structure were observed. EDX and XRD results indicated that the NaCl and Na2CO3 phases had negligible solid solubility and were essentially pure.
(4) Fracture surfaces of NaCl-10mol%Na2CO3 showed cleavage of primary NaCl and fracture through the eutectic. Crack propagation through the eutectic was deflected by the primary phase, contributing to increased strength. Similarly, in NaCl-70mol%Na2CO3, fracture surfaces showed primary Na2CO3 and the eutectic structure, suggesting a similar strengthening mechanism was operative. The fine eutectic structure also contributed to strength by reducing stress concentrations.
8. References:
- 1) T. Sakoda and T. Suzuki, U. S. Patent No. 3, 963, 818 (Jun. 15, 1976)
- 2) T. Manabe, M. Nitta and M. Yaguchi : SOKEIZAI, no. 12, vol. 44, pp. 26-30 (2003)
- 3) Yamazaki, A. Takai, O. Murakami, M. Kawabata, O. Ito and M. Kawabata : SAE Technical Paper 2004-01-1447
- 4) R. Izawa, T. Takayama, Y. Mizukusa and T. Komazaki : Report of Japan Die Casting Association, JD02 (2002) 223
- 5) J. Yaokawa, K. Anzai, Y. Yamada, H. Yoshii and H. Fukui : J. JFS 76 (2004) 823
- 6) J. Yaokawa, T. Sawada, K. Anzai, Y. Yamada, H. Yoshii and H. Fukui : J. JFS 78 (2006) 59
- 7) Y. Utsu, Japanese Patent Publication No. 52-10803 (Mar. 26, 1977)
- 8) R. W. Foreman, U. S. Patent No. 4, 840, 219 (Jun. 20, 1989)
- 9) Y. Kaneko and A. Morita : Report of Japan Die Casting Association, K71 (1971) 45
- 10) General editors, L. P. Cook and H. F. McMurdie : Phase diagrams for ceramists vol. 7, figure 6976, 7058, 7060, 7256 (Columbus, Ohio : American Ceramic Society) (1989) 16
- 11) S. Pehkonen : J. Phys. D : Appl. Phys. 6 (1973) 544
- 12) Y. Kagawa and H. Hatta : Ceramic Matrix Composites-Tailoring Ceramic Composites, (Agune Shohusha) (1990) 124
- 13) C. Gandhi and M. F. Ashby : Acta Metall. 27 (1979) 1565
9. Copyright:
- This material is a paper by "Jun Yaokawa, Daisuke Miura, Koichi Anzai, Youji Yamada and Hiroshi Yoshii". Based on "Mechanical Properties of Salt Core Comprised of Alkali Carbonate and Alkali Chloride".
- Source of the paper: https://doi.org/10.11279/jfes.78.516
This material is summarized based on the above paper, and unauthorized use for commercial purposes is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.