This introductory paper is the research content of the paper "MEASURED SF₆ EMISSIONS FROM MAGNESIUM DIE CASTING OPERATIONS" published by TMS(The Minerals, Metals & Materials Society).
1. Overview:
- Title: MEASURED SF₆ EMISSIONS FROM MAGNESIUM DIE CASTING OPERATIONS
- Author: Scott Bartos, Jerry Marks, Ravi Kantamaneni and Curtis Laush
- Publication Year: 2003
- Published Journal/Society: PRESENTED AT THE 132ND TMS ANNUAL MEETING
- Keywords: sulfur hexafluoride (SF₆), magnesium, die casting, emissions, greenhouse gas, melt protection
2. Abstract
Industrial molten magnesium processes primarily utilize sulfur hexafluoride (SF₆) as a cover gas to inhibit surface oxidation, which can result in fires if not controlled. Recent concerns with global warming and subsequent research has determined SF₆ to be a potent and long lived greenhouse gas. Current Intergovernmental Panel on Climate Change (IPCC) Good Practice Guidance for estimating the emissions of SF₆ from industrial magnesium processes assumes that 100 percent of the gas utilized for a given process is emitted directly to the atmosphere. This study examined the SF₆ utilization for a magnesium die-casting process and determined that, for the specific operating conditions, the level of SF₆ destruction was process dependent. Maximum SF₆ destruction was observed to occur during ingot feeding operations, and ranged from 16 to 20 percent. During non-feeding periods the level of destruction decreased to approximately half that observed during feeding periods. While this paper is limited to one facility using a hot-chambered die-casting unit, it does provide a useful step towards defining the true nature of SF₆ emissions from the magnesium industry as a whole.
3. Research Background:
Background of the research topic:
Molten magnesium has violent oxidative properties. The magnesium industry primarily uses sulfur hexafluoride (SF₆) to prevent oxidation and surface burning of the molten metal. SF₆ is used as a source for fluorine species, which are a component in the formation of a dense protective film on the molten magnesium surface (2).
Status of previous research:
- Current Intergovernmental Panel on Climate Change (IPCC) assumes that 100 percent of the SF₆ utilized during melt protection operations is emitted to atmosphere (5).
- Past measurement studies have been conducted to review the protection mechanism provided by SF₆. In 1972, X-ray diffraction identified several potential SF₆ by-products, including sulfur dioxide (SO₂), and magnesium fluoride (MgF₂) (8).
- In 1977, work performed at the Magnesium Research Center at Battelle's Columbus Laboratories. The study reported that the SF₆ concentration at the metal surface was about half that delivered by the metering system, which could indicate a chemical decomposition of SF₆(9).
- Additional work using cover gas mixtures containing SF₆, carbon dioxide (CO₂) and air, identified the presence of both carbon monoxide (CO) and hydrogen fluoride (HF)(10).
- A recent study, It is assumed that the general mechanism for SF₆ melt protection stems from the dissociation of SF₆ to highly reactive fluorine species, such as F or F₂. These species diffuse through the porous magnesium oxide (MgO) layer to form a dense protective film containing MgF₂.(11).
Need for research:
- Expert opinion speculates that during melt protection some form of SF₆ destruction, via chemical reaction, may occur.
- The measurement study described herein provides an initial step towards quantifying the destruction rates of SF₆ during magnesium production and processing operations.
- Since industry opinion on the extent of SF₆ destruction during magnesium production and processing differs significantly, measurements of SF₆ emissions during these operations are required to establish more accurate emission factors.
4. Research purpose and research question:
Research purpose:
- To quantify the destruction rates of SF₆ during magnesium production and processing operations.
- To difine the true nature of SF₆ emissions from the magnesium industry.
Core research:
- To identify the extent of SF₆ destruction occurring at molten magnesium holding furnaces on two hot-chambered die casting machines.
5. Research methodology
- Research Design: Quantitative experimental measurements.
- Data Collection:
- Continuous and real-time measurements were made using extractive Fourier Transform Infrared (FTIR) spectroscopic systems from MKS Instruments.
- Gas grab samples were taken from the exhaust stream of the FTIR via one-milliliter syringe pulls, and manually injected into a HP 5890A gas chromatograph (GC).
- Analysis Method:
- FTIR: Real-time monitoring of gas stream from a Monel sample probe. Instrumental resolution was set to 0.5 cm¯¹ and signal averaging was performed over one or two-minute periods.
- GC: Used a thermal conductivity detector (TCD) and a HayeSep Q 80/100 column. Helium was used as the carrier gas. The gas chromatograph was calibrated with a multi-point calibration curve prior to use each day.
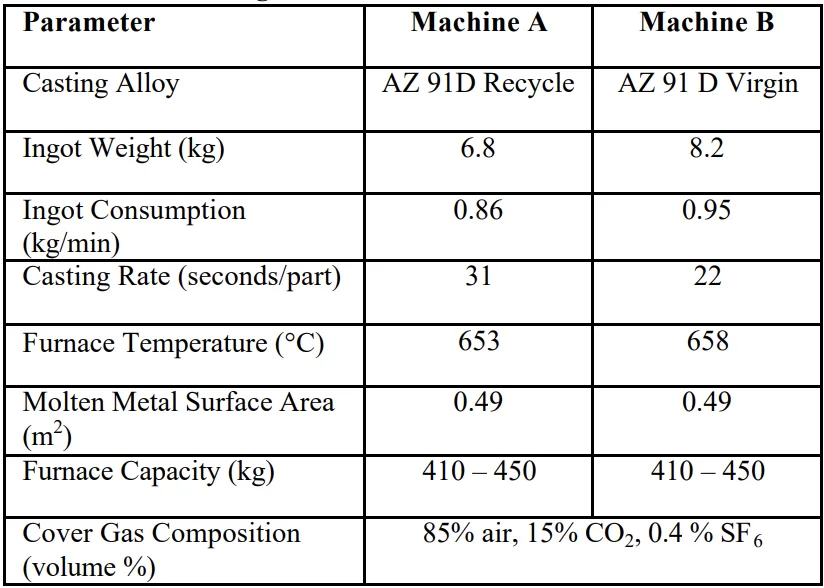
- Research Scope: Two hot-chambered die casting machines (Machine A and B) located at the Product Technologies Inc. facility in Minnesota. The machines consisted of a holding furnace assembly for the molten magnesium, pneumatic injection piston, and die press apparatus.
6. Key research results:
Key research results and presented data analysis:
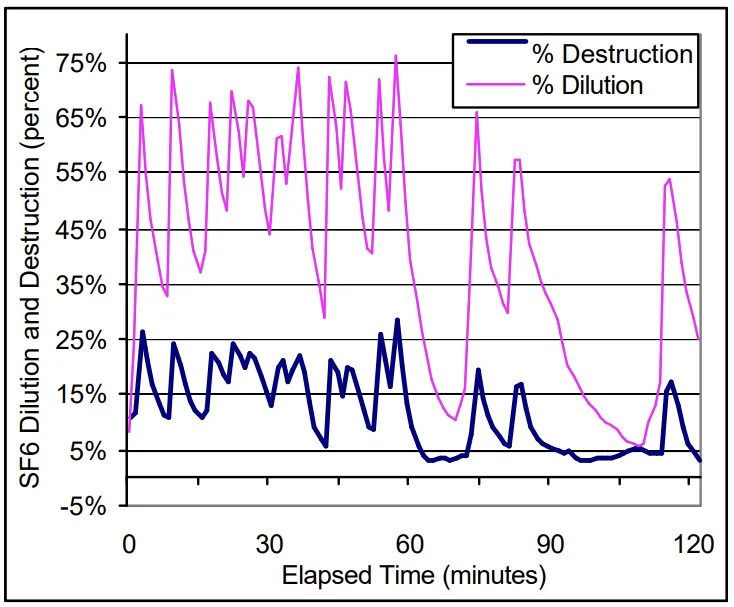
- The overall average SF₆ destruction, based on the specific operating conditions and neglecting observed process variations, was determined to be on the order of 10 percent.
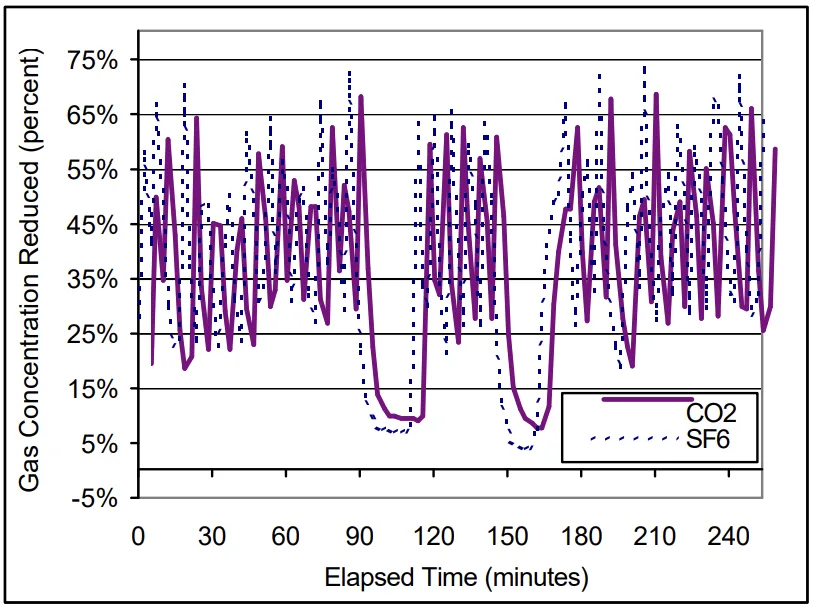
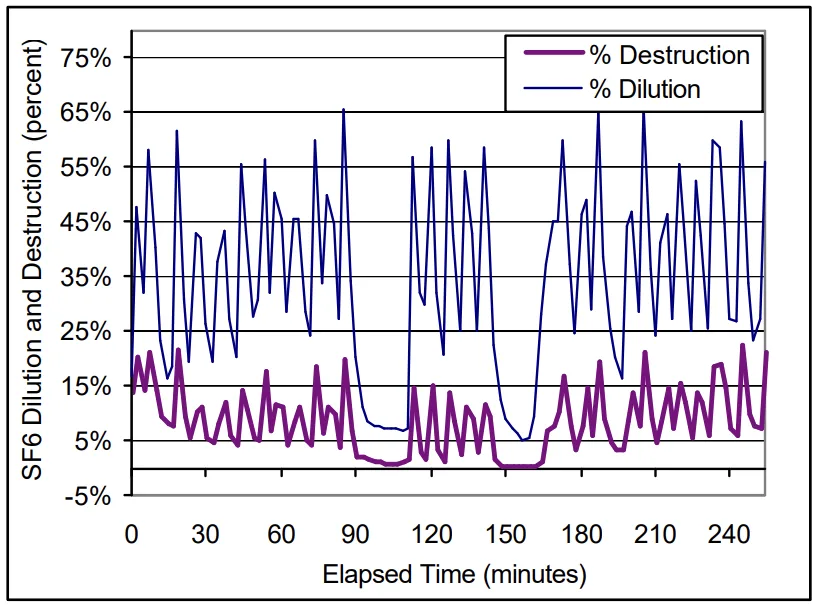
- For Machine A, SF₆ destruction ranged from 6.5 to 16 percent, while Machine B ranged from 12.5 to 20 percent.
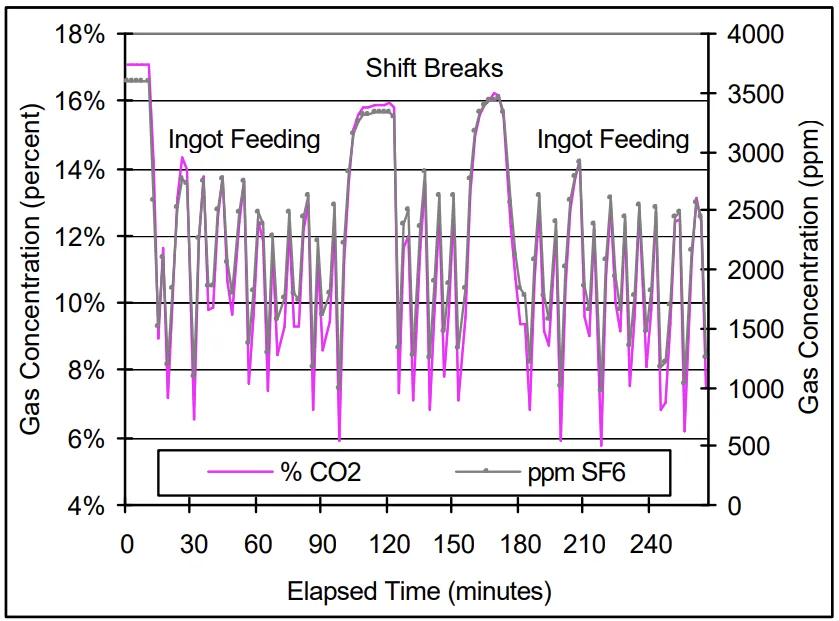
- Maximum SF₆ destruction was observed to occur during the moments following ingot feeding. During this period the destruction of SF₆, for both machines, was observed to be approximately 16-20 percent.
- During non-feeding periods, SF₆ destruction was on average 6.5 and 12.5 percent for Machines A and B, respectively.
- When the casting machine was not operating, the overall destruction of SF₆ decreased to minimal levels, approximately 0.6 to 1.4 percent.
- FTIR sampling identified two gaseous reaction byproducts, HF and SO₂.
List of figure names:
- Figure 1. Cover Gas CO₂, H₂O and SF₆ Concentrations: Machine A
- Figure 2. Percent Cover Gas CO₂ and SF₆ Reduction: Machine A
- Figure 3. SF₆ Dilution and Destruction: Machine A
- Figure 4. SF₆ Dilution and Destruction: Machine B
7. Conclusion:
Summary of key findings:
The level of SF₆ destruction was strongly correlated to die casting process operations. During ingot feeding operations, 6 to 8 kg ingots are added to the melt furnace and, consequently, break the in-situ SF₆ protective film. During this heightened melt surface turbulence, SF₆ destruction reached a maximum(16-20 percent). Since the level of SF₆ destruction dropped to approximately 1 percent when the casting machine was not operating, it can be concluded that a minimum level of SF₆ destruction occurs during casting operations. This average minimum level was observed to be 6.5 and 12.5 percent for Machines A and B, respectively.
Summary of research results. Academic significance of the research, practical implications of the research
- This study provides the specific operating conditions, the level of SF₆ destruction was process dependent.
- It is assumed that all SF₆ decomposition occurs at the melt surface, without the release of gaseous byproducts.
- This paper is a useful step toward defining the true nature of SF₆ emissions from the magnesium industry.
8. References:
- Roskill Consulting Group, The Economics of Magnesium Metal, 2001.
- Tranell, G., et al., “A Systematic Approach for Identifying Replacements to SF6/SO2 in the Magnesium Industry – An IMA / SINTEF-NTNU Cooperative Project," Proceedings of the 57th Annual International Magnesium Association Conference, May 2001.
- Hanawalt, J.D., “Practical Protective Atmospheres for Molten Magnesium," Metals Engineering Quarterly, November 1972. pp. 6.
- IPCC, Climate Change 2001: The Scientific Basis, Intergovernmental Panel on Climate Change, Third Assessment Report, 2001.
- IPCC, Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories, Section 3.4, SF 6 Emissions from Magnesium Production, http://www.ipcc-nggip.iges.or.jp/public/gp/pdf/3_Industry.pdf
- Bartos, S., "Building a Bridge for Climate Protection: U.S. EPA and the Magnesium Industry,” Proceedings of the 59th Annual International Magnesium Association Conference, May 2003.
- U.S. EPA. Inventory of Greenhouse Gas Emissions and Sinks: 1990-2000, ΕΡΑ 236-R-01-001, April 2002.
- Hanawalt, J. D., "Practical Protective Atmospheres for Molten Magnesium," Metals Engineering Quarterly, pp 6 – 10, November 1972.
- Couling, S., Bennett, F., Leontis, T., "Fluxless Melting of Magnesium," Light Metals, pp 545 – 560, 1977.
- Couling, S., Leontis, T., “Improved Protection of Molten Magnesium with Air/CO2/SF6 Gas Mixtures," Light Metals, pp 997-1009, 1980
- Tranell, G., G. Pettersen, K. Aastad, T. A. Engh, I. Solheim, M. Syvertsen, B. Oye, “A Systematic Approach for Identifying Replacements to SF 6/SO2 in the Magnesium Industry - An IMA / SINTEF-NTNU Cooperative Project," 57th Anuual IMA Conference Proceedings, May 2001.
9. Copyright:
- This material is a paper by "Scott Bartos, Jerry Marks, Ravi Kantamaneni and Curtis Laush": Based on "MEASURED SF₆ EMISSIONS FROM MAGNESIUM DIE CASTING OPERATIONS".
- Source of paper: Not provided in the OCR text.
This material was created to introduce the above paper, and unauthorized use for commercial purposes is prohibited. Copyright © 2025 CASTMAN. All rights reserved.