This introduction paper is based on the paper "Mechanism and performance of coal spontaneous combustion with a halide carrier inorganic salt inhibitor" published by "Chinese Journal of Engineering".
1. Overview:
- Title: Mechanism and performance of coal spontaneous combustion with a halide carrier inorganic salt inhibitor
- Author: ZHANG Yan-ni, HOU Yun-chao, LIU Bo, DENG Jun, LIU Chun-hui, YANG Jing-jing, WEN Xin-yu
- Year of publication: 2021
- Journal/academic society of publication: Chinese Journal of Engineering
- Keywords: halide carrier inorganic salt; inhibitor; coal spontaneous combustion; differential scanning calorimetry; apparent activation energy
2. Abstract:
Coal spontaneous combustion seriously restricts the safe production of coal mines, and adding an inhibitor is one of the effective methods to prevent coal spontaneous combustion. To improve the pertinence and high efficiency of the inhibitor, this paper considered the intrinsic properties and external conditions that affect the occurrence of coal spontaneous combustion, combined with the characteristics that the rare earth hydrotalcite can effectively improve the thermal stability, coupling, and flame retardancy of the coal and the halide inhibitor. The halide inhibitor can enhance the permeability, dispersion, and uniformity of the rare earth hydrotalcite as a carrier. The halide carrier inorganic salt inhibitor was prepared. To study the inhibition mechanism and performance of the halide carrier inorganic salt inhibitor on coal spontaneous combustion, differential scanning calorimetry (DSC) was used to test the variation law of parameters, such as stage characteristics, characteristic temperature, thermal effect, and apparent activation energy in the process of coal spontaneous combustion under the action of a rare earth hydrotalcite, MgCl2 and a halide carrier inorganic salt inhibitor. Test results reveal that the OH of the rare earth hydrotalcite laminate can generate a weak hydrogen bond with acidic functional groups such as –COOH in coal molecules so that the activity of the acidic functional groups is weakened. Mg²⁺ complexes with –COO⁻ in coal molecules to form –COOMg–, resulting in the weakening of the C=O activity in –COO⁻, which is the main mechanism of the halide carrier inorganic salts inhibiting coal spontaneous combustion. The endothermic peak of the DSC curve appears as a double peak or multi-peak after the addition of halide carrier inorganic salts to the coal sample. Compared with the raw coal, the peak temperature is shifted back by 50–60 °C, the T₁ temperature is shifted back by 90–100 °C, and the total heat release decreased by 19–27 kJ·g⁻¹. Furthermore, the apparent activation energy of each stage of the coal body is effectively improved. Results revealed that the halide carrier inorganic salt inhibitor could effectively inhibit the reaction process of coal spontaneous combustion.
3. Introduction:
Coal is a primary energy source, and its production and consumption are significant globally. However, approximately 75% of coal seams in China are prone to spontaneous combustion, leading to substantial resource loss and CO2 emissions. Developing effective inhibitors is crucial for preventing thermokinetic disasters in coal mines and ensuring safe production.
Current inhibitors mainly include halide salts, inert gases, and polymer emulsions. These can be classified based on their mechanism into chemical inhibition and physical inhibition. Physical inhibition controls combustibles or ignition sources, while chemical inhibition acts at a microscopic level by destroying or capturing active functional groups in coal molecules, thereby slowing down the coal-oxygen recombination process.
Halide inhibitors are widely used due to their good coating and water-absorbing properties. However, they have disadvantages such as requiring large amounts, causing corrosion to equipment, and having short inhibition times. Rare earth hydrotalcites, as functional materials, offer good thermal stability, ion exchangeability, and tunable layer composition, making them suitable for various applications. Adding rare earth hydrotalcite to halide inhibitors can enhance the thermal stability, coupling, and flame retardancy of the coal-inhibitor system. Conversely, using halide inhibitors as carriers can overcome the poor permeability, dispersion, and uniformity of rare earth hydrotalcites. This study focuses on preparing a halide carrier inorganic salt inhibitor using rare earth hydrotalcite and MgCl2, and investigating its inhibition characteristics and mechanism through theoretical analysis and experimental testing, aiming to provide fundamental data for developing new and efficient coal spontaneous combustion inhibitors.
4. Summary of the study:
Background of the research topic:
Spontaneous combustion of coal is a significant safety hazard in coal mining operations, leading to resource loss and environmental pollution. The application of inhibitors is a key strategy to prevent such incidents.
Status of previous research:
Various inhibitors, including halide salts, inert gases, and polymer emulsions, have been developed. Halide salts are common but suffer from drawbacks like high dosage, corrosivity, and short-term effectiveness. Rare earth hydrotalcites have shown promise in improving thermal stability and flame retardancy. Previous studies have explored individual components like MgCl2 and composite inhibitors, indicating the potential for synergistic effects.
Purpose of the study:
This study aimed to develop a novel halide carrier inorganic salt inhibitor by combining rare earth hydrotalcite with MgCl2. The research focused on understanding the inhibition mechanism and performance of this composite inhibitor on coal spontaneous combustion, with the goal of providing a basis for creating more efficient and targeted inhibitors.
Core study:
The core of the study involved the preparation of a halide carrier inorganic salt inhibitor using rare earth hydrotalcite and MgCl2. The inhibitory effects were evaluated by applying these inhibitors to coal samples. Differential scanning calorimetry (DSC) was employed to analyze the thermal behavior, including characteristic temperatures, heat effects, and apparent activation energies during coal oxidation. Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) were used for microstructural and elemental analysis of the treated coal samples.
5. Research Methodology
Research Design:
The research employed an experimental design. It involved the synthesis of rare earth hydrotalcite, preparation of different inhibitor formulations (rare earth hydrotalcite alone, MgCl2 alone, and their combination as a halide carrier inorganic salt inhibitor), and treatment of coal samples with these inhibitors. The performance of the inhibitors was then evaluated through thermal analysis and microstructural characterization.
Data Collection and Analysis Methods:
- Materials: Bin-chang non-stick coal was used as the research object. Rare earth hydrotalcite was synthesized using a co-precipitation method. MgCl2 was used as another inhibitor component.
- Sample Preparation: Coal samples (0.105–0.15 mm) were mixed with different inhibitor solutions (compositions detailed in Table 2 of the original paper) and dried.
- Differential Scanning Calorimetry (DSC): A NETZSCH DSC200F3 instrument was used. 10 mg samples were tested in an air atmosphere (25 mL·min⁻¹) with a heating rate of 5 °C·min⁻¹ from 30 °C to 450 °C.
- Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS): A QUANTA FEG-450 SEM with a MAX-50 EDS attachment was used to observe the micro-morphology and elemental composition of the samples.
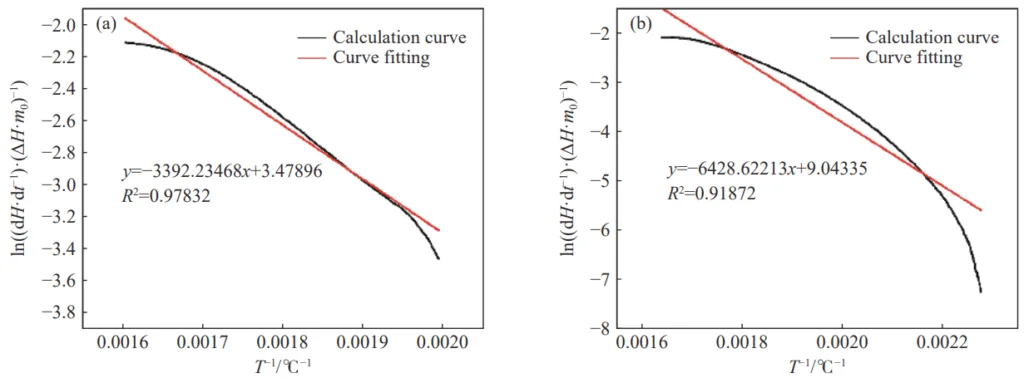
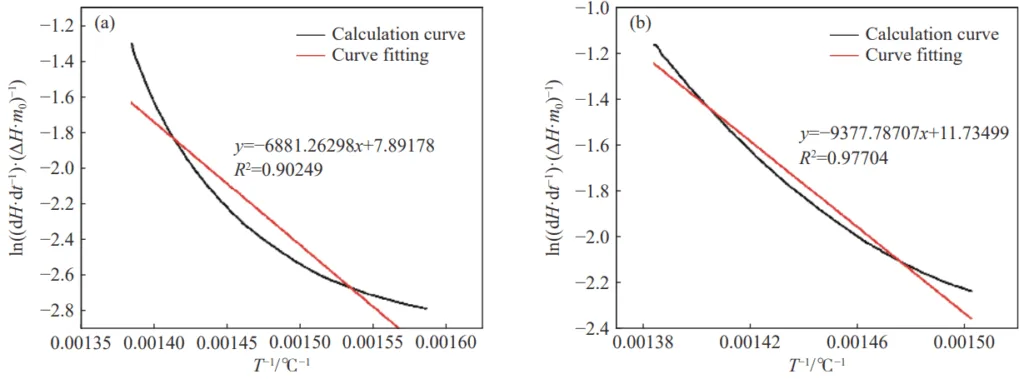
- Kinetic Analysis: The apparent activation energy (E) for different stages of coal oxidation was calculated from DSC data using the Arrhenius equation and a derived kinetic model (Equation 5 in the original paper).
Research Topics and Scope:
- Investigation of the micro-morphology and elemental distribution of coal samples treated with different inhibitors.
- Analysis of the heat release characteristics (stage characteristics, characteristic temperatures, thermal effects) of coal spontaneous combustion under the influence of rare earth hydrotalcite, MgCl2, and the halide carrier inorganic salt inhibitor.
- Elucidation of the inhibition mechanism of the halide carrier inorganic salt inhibitor.
- Determination of the apparent activation energy for different stages of the coal oxidation process with and without inhibitors.
6. Key Results:
Key Results:
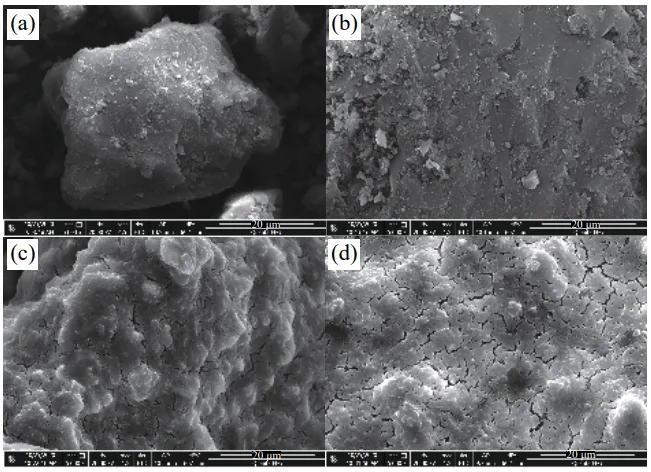
- Microstructure and Composition (SEM/EDS): The chemical co-precipitation method effectively improved the composite degree of the inhibitor with coal. MgCl2, acting as a carrier, enhanced the penetration, dispersion, and uniformity of the rare earth hydrotalcite on the coal surface.
- Thermal Behavior (DSC):
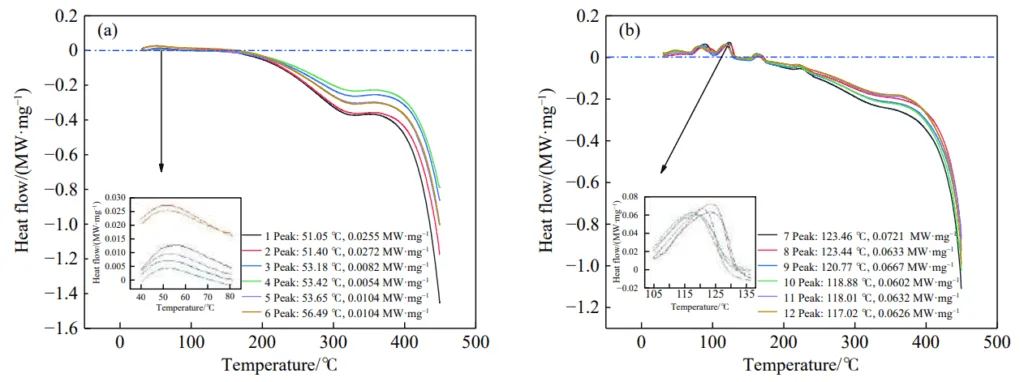
- The addition of the halide carrier inorganic salt inhibitor to coal samples resulted in the appearance of double or multiple endothermic peaks in the DSC curves.
- Compared to raw coal, the peak temperature of the endothermic process was shifted to higher temperatures by 50–60 °C.
- The initial temperature of significant heat release (T₁ temperature) was shifted to higher temperatures by 90–100 °C.
- The total heat released during oxidation was reduced by 19–27 kJ·g⁻¹.
- Inhibition Mechanism:
- The -OH groups on the laminate of the rare earth hydrotalcite can form weak hydrogen bonds with acidic functional groups (e.g., -COOH) in coal molecules, thereby reducing the activity of these acidic groups.
- Mg²⁺ ions from MgCl2 can form complexes with -COO⁻ groups in coal (forming -COOMg-), which weakens the C=O bond activity within the -COO⁻ group. This is a primary mechanism for the halide carrier inorganic salt inhibiting coal spontaneous combustion.
- The halide carrier inorganic salt inhibitor demonstrated both physical (e.g., coating, moisture retention by MgCl2) and chemical inhibition effects, delaying the heat absorption phase, increasing the amount of heat absorbed, and significantly reducing heat release in subsequent oxidation stages.
- Apparent Activation Energy: The addition of inhibitors, particularly the halide carrier inorganic salt inhibitor, effectively increased the apparent activation energy for both the slow and rapid heat release stages of coal oxidation, indicating a higher energy barrier for combustion and thus better inhibition.
Figure Name List:
- Fig.1 Test sample SEM: (a) sample 1; (b) sample 2; (c) sample 7; (d) sample 8
- Fig.2 Test sample EDS: (a) sample 1; (b) sample 2; (c) sample 7; (d) sample 8
- Fig.3 Curve of the heat release rate of the test sample: (a) sample 1–6; (b) sample 7–12
- Fig.4 Heat release rate curve: (a) sample 1; (b) sample 2; (c) sample 3; (d) sample 4; (e) sample 5; (f) sample 6; (g) sample 7; (h) sample 8; (i) sample 9; (j) sample 10; (k) sample 11; (l) sample 12
- Fig.5 Apparent activation energy curve of the test sample during the slow heat release stage: (a) sample 1; (b) sample 7
- Fig.6 Apparent activation energy curve of the test sample during the rapid heat release stage: (a) sample 1; (b) sample 7
7. Conclusion:
The study yielded the following main conclusions:
- Microscopic analysis (SEM/EDS) confirmed that the chemical co-precipitation method enhances the integration of the inhibitor with the coal. MgCl2 acts as an effective carrier, improving the penetration, dispersion, and uniformity of the rare earth hydrotalcite. DSC analysis revealed distinct thermal reaction stages (heat absorption, slow heat release, rapid heat release) and highlighted differences in the thermal reactivity and inhibition mechanisms of various inhibitors.
- The halide carrier inorganic salt inhibitor exerts a dual inhibition effect (physical and chemical) on coal spontaneous combustion. This is macroscopically manifested by a delay in characteristic temperatures (peak endothermic temperature and T₁), the appearance of multiple absorption peaks, prolongation of the heat absorption phase with increased heat absorption, and a significant reduction in heat release during the various oxidation stages.
- Rare earth hydrotalcite effectively enhances the inhibition efficiency of halide salts, suppresses heat accumulation in coal, delays the onset of spontaneous combustion, and significantly increases the apparent activation energy of coal in different oxidation stages. Therefore, the halide carrier inorganic salt inhibitor can effectively suppress the coal spontaneous combustion process, offering a promising approach for developing more efficient inhibitors.
8. References:
- [1] Zhang Y N, Chen L, Zhao J Y, et al. Evaluation of the spontaneous combustion characteristics of coal of different metamorphic degrees based on a temperature-programmed oil bath experimental system. J Loss Prev Process Ind, 2019, 60: 17
- [2] Zhang Y N, Hou Y C, Zhao J Y, et al. Heat release characteristic of key functional groups during low-temperature oxidation of coal. Combust Sci Technol, 2020: 1
- [3] Zhao J Y, Deng J, Chen L, et al. Correlation analysis of the functional groups and exothermic characteristics of bituminous coal molecules during high-temperature oxidation. Energy, 2019, 181: 136
- [4] Guo W J. Experimental test and examination of the influential factors of the coal spontaneous combustion. J Saf Environ, 2018, 18(4): 1307 (郭文杰. 煤自燃特性影响因素的试验研究. 安全与环境学报, 2018, 18(4): 1307)
- [5] Zhu H T, Li Y N, Zhai Q Y, et al. Preparation and performance research of hydrotalcites containing rare earth element. Henan Chem Ind, 2019, 36(7): 15 (朱洪涛, 李岩娜, 翟秋月, 等. 稀土元素类水滑石的制备及其性能研究. 河南化工, 2019, 36(7): 15)
- [6] Liu Y Z, Duan T X, Deng Q, et al. Preparation of calcined hydrotalcite and adsorption of Cr(VI). Guangzhou Chem Ind, 2020, 48(15): 109 (刘奕祯, 段天欣, 邓仟, 等. 焙烧水滑石的制备及其吸附 Cr(VI)的研究. 广州化工, 2020, 48(15): 109)
- [7] Pang Y Q, Zhang Q. Research of inhibitor suppression on coal spontaneous combustion by magnesium chloride. Datong Coal Sci Technol, 2020(3): 51 (庞叶青, 张奇. 阻化剂氯化镁抑制煤自燃的实验研究. 同煤科技, 2020(3): 51)
- [8] Xu H Y. Experimental study of thermogravimetric kinetics on the composite inhibitor for inhibiting coal spontaneous combustion. Min Res Dev, 2019, 39(6): 79 (许红英. 复合阻化剂抑制煤自燃的热重动力学实验研究. 矿业研究与开发, 2019, 39(6): 79)
- [9] Ma D J, Tang Y B. Influence of associated metal elements in coal on low-temperature oxidation characteristics of coal. Coal Sci Technol, 2019, 47(2): 203 (马冬娟, 唐一博. 煤中伴生金属元素对煤低温氧化特性的影响. 煤炭科学技术, 2019, 47(2): 203)
- [10] Wang F S, Wang J T, Dong X W, et al. Experimental research on resistance characteristics of hypophosphite to coal spontaneous combustion. Saf Coal Mines, 2020, 51(5): 45 (王福生, 王建涛, 董宪伟, 等. 次磷酸盐对煤自燃的阻化特性实验研究. 煤矿安全, 2020, 51(5): 45)
- [11] Jin Y F, Li Y H, Liu B. Research on inhibiting effects of LDHs on coal spontaneous combustion. Coal Technol, 2017, 36(10): 101 (金永飞, 李毅恒, 刘博. 稀土类水滑石的煤自燃阻化效果研究. 煤炭技术, 2017, 36(10): 101)
- [12] Yang J X, Bai Z J. Experimental study on inhibition characteristic of LDHs inhibitor to bituminous coal. Saf Coal Mines, 2018, 49(8): 35 (杨计先, 白祖锦. LDHs阻化剂对烟煤的阻化特性实验研究. 煤矿安全, 2018, 49(8): 35)
- [13] Zhou X, Yuan H K, He P, et al. Effect of surface modifier on properties of Zn-Mg-Al hydrotalcites as heat stabilizer. Fine Chem, 2018, 35(8): 1389 (周喜, 袁浩坤, 何鹏, 等. 表面改性剂对Zn-Mg-Al水滑石热稳定剂性能影响. 精细化工, 2018, 35(8): 1389)
- [14] Wu W R, Liu T, Liu Z H, et al. Research progress of coal self ignition inhibitor. Appl Chem Ind, 2017, 46(2): 356 (武卫荣, 刘涛, 刘振辉, 等. 煤自燃阻化剂的研究进展. 应用化工, 2017, 46(2): 356)
- [15] Zhang Y T, Shi X Q, Li Y Q, et al. Inhibiting effects of Zn/Mg/Al layer double hydroxide on coal spontaneous combustion. J China Coal Soc, 2017, 42(11): 2892 (张玉涛, 史学强, 李亚清, 等. 锌镁铝层状双氢氧化物对煤自燃的阻化特性. 煤炭学报, 2017, 42(11): 2892)
- [16] Li J H, Wang B, Zhang A S, et al. Research on inhibition effect of halogen inhibitor on coal seam of Guotun Coal Mine. Coal Chem Ind, 2020, 43(5): 142 (李进海, 王兵, 张安山, 等. 卤盐阻化剂对郭屯煤矿煤层的阻化效果研究. 煤炭与化工, 2020, 43(5): 142)
- [17] Dong X W, Ai Q X, Wang F S, et al. Research on thermal characteristics in the process of coal oxidation inhibition. J Saf Sci Technol, 2016, 12(4): 70 (董宪伟, 艾晴雪, 王福生, 等. 煤氧化阻化过程中的热特性研究. 中国安全生产科学技术, 2016, 12(4): 70)
- [18] Zhou K Q, Gao R, Qian X D. Self-assembly of exfoliated molybdenum disulfide (MoS2) nanosheets and layered double hydroxide (LDH): towards reducing fire hazards of epoxy. J Hazard Mater, 2017, 338: 343
- [19] Wu B, Lou P, Wang C. Analysis of coal spontaneous combustion control by inhibitor. China Coal, 2014, 40(6): 117 (吴兵, 娄鹏, 王超. 阻化剂防治煤自燃效果分析. 中国煤炭, 2014, 40(6): 117)
- [20] Zheng L F. Test and analysis on salty retardants performance to restrain coal oxidized spontaneous combustion. Coal Sci Technol, 2010, 38(5): 70 (郑兰芳. 抑制煤氧化自燃的盐类阻化剂性能分析. 煤炭科学技术, 2010, 38(5): 70)
- [21] Li X P, Chen Y G, Zhang J S, et al. Study on the inhibition effect of chlorine salt composite inhibitor on spontaneous combustion of different coal samples. Coal Eng, 2020, 52(2): 106 (李绪萍, 陈映光, 张金山, 等. 氯盐复合阻化剂对不同煤样自燃阻化效果的研究. 煤炭工程, 2020, 52(2): 106)
- [22] Yang Y. Mechanism and Performance of Inhibitor Based on Oxidation Characteristic of the Spontaneous Combustion of Coal [Dissertation]. Xi’an: Xi’an University of Science and Technology, 2015 (杨漪. 基于氧化特性的煤自燃阻化剂机理及性能研究[学位论文]. 西安: 西安科技大学, 2015)
- [23] Zhang X H, Ding F, Zhang Y T, et al. Experimental study on LDHs composite inhibitor to coal resistance property. Coal Sci Technol, 2017, 45(1): 84 (张辛亥, 丁峰, 张玉涛, 等. LDHs复合阻化剂对煤阻化性能的试验研究. 煤炭科学技术, 2017, 45(1): 84)
- [24] Duan Z Y, Wang F. Influence of MgCl2 on initial oxidation and secondary oxidation of coal. Saf Coal Mines, 2017, 48(6): 13 (段志勇, 王飞. MgCl2对煤一次氧化与二次氧化影响的实验研究. 煤矿安全, 2017, 48(6): 13)
- [25] Zhao J Y, Zhang Y L, Deng J, et al. Key functional groups affecting the release of gaseous products during spontaneous combustion of coal. Chin J Eng, 2020, 42(9): 1139 (赵婧昱, 张永利, 邓军, 等. 影响煤自燃气体产物释放的主要活性官能团. 工程科学学报, 2020, 42(9): 1139)
9. Copyright:
- This material is a paper by "ZHANG Yan-ni, HOU Yun-chao, LIU Bo, DENG Jun, LIU Chun-hui, YANG Jing-jing, WEN Xin-yu". Based on "Mechanism and performance of coal spontaneous combustion with a halide carrier inorganic salt inhibitor".
- Source of the paper: https://doi.org/10.13374/j.issn2095-9389.2020.12.25.001
This material is summarized based on the above paper, and unauthorized use for commercial purposes is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.