This introduction paper is based on the paper "THE ROLE OF THERMAL PROCESSES IN THE FORMATION OF GALVANIC ZINC ANODES PROPERTIES"
1. Overview:
- Title: THE ROLE OF THERMAL PROCESSES IN THE FORMATION OF GALVANIC ZINC ANODES PROPERTIES
- Author: Prof. Vladimir Kechin¹, Prof. Efim Lyublinski², Phd. Evgeny Prusov¹ (¹Vladimir State University, Russia, kechin@vlsu.ru, ²COR/SCI, LLC, USA, elyublinski@gmail.com)
- Year of publication: Not explicitly stated in the paper.
- Journal/academic society of publication: Not explicitly stated in the paper.
- Keywords: sacrificial anodes, structure, foundry, electrochemistry
2. Abstract:
The main reasons for the appearance of electrochemical heterogeneity of cast sacrificial galvanic anodic alloys in the "metal-electrolyte" system are internal factors related to the nature of the metal, its composition, structure, etc. Obviously, when developing the technology for manufacturing of cast anodes, special attention should be paid to ensuring the structural homogeneity of the alloys. The main role in the formation of the structure and basic properties of cast anodes is played by thermal processes that affect the conditions for solidification of the melt in the mold. The results of studies of the structure and basic electrochemical properties of cast zink sacrificial alloys (ZSA) depending on the cooling conditions are presented in this paper. The analysis of the temperature fields of the solidifying metal (when the metal is cooled) and the shape (when the mold is heated) at different cooling intensities has made it possible to optimize the duration of the casting cycle. This takes into account the cooling conditions determined by the initial temperature of the mold, which ensures a homogeneous structure and stable electrochemical properties of the material over the entire thickness of the tread. For example, it is established that for the casting of zinc alloys treads weighing 18 kg, the temperature of the mold before casting should be 120-160°C. In these conditions, the necessary quality of cast sacrificial anodic alloys is achieved Current Capacity-Efficiency 93-96%; Corrosion Potential -E = 815-820 mV vs. to SHE. The required duration of the casting cycle is 10-14 min. Similar data was obtained for sacrificial anodes of various shapes and sizes. Based on the results of studies of the thermal interaction of zinc anodes with a casting mold (sandy-argillaceous, cast iron and steel water-cooled) using numerical simulation methods, the expediency of casting anodes into water-cooled forms is provided, providing the most favorable conditions for heat removal and obtaining a homogeneous structure of cast treads. Based on the results obtained, a technology has been developed for casting zinc anodes of various sizes, which provides high and stable electrochemical properties.
3. Introduction:
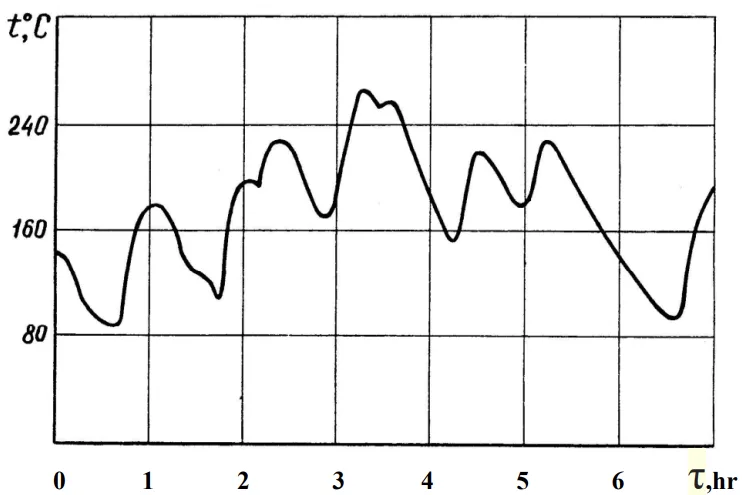
The thermal processes play a leading role in the formation of most properties of castings during solidification of the melt [1-11]. Therefore, studying the conditions for solidification of the melt in a mold is the most important task of the thermal theory of casting. The urgency of this issue is also dictated by the fact that when casting the sacrificial anodes (SA), there are significant temperature fluctuations of metallic forms [12]. Thus, continuous monitoring of temperature on one of the series cast iron molds during the casting of zinc SA [following composition ZSA1: Zn+(0,4-0,6%)Al and ZSA2: Zn+(0,5-0,7%)Al+(0,1-0,3%Mg+0,1-0,3%Mn), contaminants less than: Fe-0,0015%, Cu-0,001%, Pb-0,005% noted that the initial temperature of the mold changes during the shift from 80 to 260°C(Fig.1). Apparently, this can explain the destabilization and reduction of the ZSA electrochemical properties that occur in the early stages of the use of SA, as well as contradictory data on the effectiveness of individual SA from one grade of alloy and even one melting [13].
4. Summary of the study:
Background of the research topic:
Thermal processes are crucial in determining the properties of castings during melt solidification. This is particularly important for sacrificial anodes (SA), where manufacturing processes, especially in metallic molds, involve significant temperature fluctuations that can affect the final product's performance.
Status of previous research:
Previous observations indicated significant temperature fluctuations (80°C to 260°C) in metallic molds during the casting of zinc sacrificial anodes (ZSA) (Fig.1). Such fluctuations are believed to cause destabilization and reduction in the electrochemical properties of ZSA, leading to contradictory data on their effectiveness, even within the same alloy grade or melt [13].
Purpose of the study:
The main purpose of this work is to study the structure and basic electrochemical properties of cast sacrificial anodes from Zn-Al alloys[13] depending on the thermal conditions of cooling.
Core study:
The study investigated the influence of thermal processes during casting on the structure and electrochemical properties of zinc sacrificial alloys (ZSA1 and ZSA2). Key aspects included:
- Analyzing temperature fields during solidification with varying initial mold temperatures (20°C, 160°C, 310°C in cast iron molds).
- Determining the nature of solidification using the pouring out method and examining macrostructure and microstructure.
- Evaluating electrochemical properties such as Current Capacity-Efficiency (CC) and Corrosion Potential (-E).
- Optimizing casting cycle duration based on mold temperature and cooling conditions.
- Comparing casting outcomes in different mold materials (sandy-argillaceous, cast iron, water-cooled steel).
- Investigating the effect of casting temperature (450°C, 500°C, 550°C) and solidification rate (Vs) on ZSA properties.
- Developing and assessing an improved mold design featuring water-cooling and thermal insulation to achieve directional solidification and enhanced anode quality.
5. Research Methodology
Research Design:
The research employed an experimental approach. This involved casting ZSA under controlled, varied thermal conditions, followed by thermal analysis, microstructural characterization, and electrochemical performance testing. Numerical simulation methods were also utilized to study the thermal interaction between the ZSA and the mold.
Data Collection and Analysis Methods:
- Thermometry: Temperature fields in the solidifying alloy and the mold were measured using thermocouples (as shown in Fig. 3), with data recorded by an automatic twelve-point potentiometer.
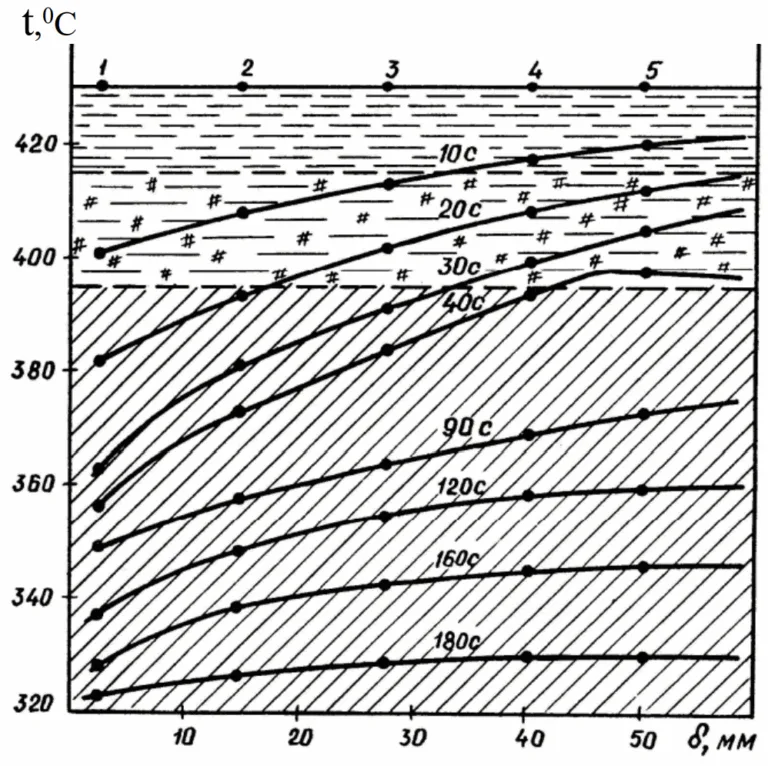
- Pouring Out Method: This method was used to study the solidification process, allowing for the measurement of the thickness and mass of the solidifying crust at different time intervals (Table 2, Fig. 5).
- Macrostructure and Microstructure Analysis: The macrostructure of ZSA cast under different conditions was examined (Fig. 2). Microstructural analysis (x100 magnification) was performed on ZSA1 and ZSA2 alloys cast at various temperatures and in different mold types (Fig. 8, Fig. 9).
- Electrochemical Property Testing: Key electrochemical properties, including Current Capacity (CC), stationary potential (-Ec or -φc), and potential under polarization (-Ep or -φp), were determined (Table 3). Tests were conducted in artificial seawater.
- Numerical Simulation: These methods were applied to confirm experimental findings regarding the benefits of casting sacrificial anodes into water-cooled molds.
Research Topics and Scope:
- The study focused on two zinc-aluminum sacrificial anode alloys: ZSA1 (Zn+(0.4-0.6%)Al) and ZSA2 (Zn+(0.5-0.7%)Al+(0.1-0.3%Mg+0.1-0.3%Mn)).
- Investigation of the effect of initial mold (cast iron) temperature (20°C, 160°C, 310°C) on solidification characteristics, microstructure, and electrochemical properties.
- Optimization of the casting cycle, considering mold heating and cooling times to maintain consistent pre-pour mold temperatures.
- Comparison of casting performance in different mold types: sandy-argillaceous (chamotte), cast iron, and water-cooled steel molds.
- Assessment of the impact of casting temperature (450°C, 500°C, 550°C) and mold material (nonmetallic vs. metallic) on CC and microstructure.
- Evaluation of the influence of solidification rate (Vs) on CC and microstructure.
- Design and testing of an improved mold for directional solidification of zinc treads (Fig. 10).
6. Key Results:
Key Results:
- Fluctuations in initial mold temperature (e.g., 80°C to 260°C during a shift, Fig. 1) significantly impact the structure and electrochemical properties of ZSA, potentially leading to reduced CC (from 96% to 80% as mold temperature changes from 20°C to 300°C).
- For casting 18 kg zinc alloy treads, an optimal initial mold temperature of 120-160°C ensures a Current Capacity-Efficiency of 93-96% and a Corrosion Potential (-E) of 815-820 mV vs. SHE, with a casting cycle of 10-14 minutes.
- ZSA macrostructure exhibits cortical, columnar, and non-oriented crystal zones; the most uniform fine-grained structure is achieved with initial mold temperatures of 20°C and 160°C (Fig. 2).
- The intensity of heat removal dictates the solidification rate and character. For ZSA treads in metallic molds, complete solidification time varied: 215s (20°C mold), 255s (160°C mold), and 440s (310°C mold) (Table 2).
- While stationary potential and potential at polarization (3 A/m²) are largely independent of solidification conditions, CC is affected: for ZSA1, CC was 92-96% (20°C mold), 90-93% (160°C mold), and 80-87% (310°C mold) (Table 3).
- Maintaining a constant initial mold temperature is crucial for steady-state casting. The optimal total casting cycle (tц) is achieved by balancing solidification/cooling time (t1) and mold cooling time (t2) (Fig. 6). For zinc treads, a mold temperature of 120-160°C is recommended.
- Increasing casting temperature from 450°C to 550°C reduces CC for both ZSA1 and ZSA2, in both nonmetallic (chamotte) and metallic (cast-iron) molds. Metallic molds generally yield higher CC (Fig. 7).
- Higher casting temperatures result in coarser microstructures and larger second-phase particles, correlating with decreased CC (Fig. 8).
- CC of ZSA alloys increases with the rate of solidification (Vs) (Fig. 9). Regression equations for ZSA1: CC = 127-0,072tc (casting temperature dependence) and CC = 84,21 + 0,035 Vculing (solidification rate dependence).
- An improved mold design (Fig. 10) with a water-cooled base, insulated side walls, and a heat-shielding screen promotes directional solidification, resulting in a homogeneous crystal structure, smooth surface, elimination of shrinkage holes, and CC of 96-98%. This design reduces the casting cycle to 2-3 minutes (Fig. 11).
Figure Name List:
- Figure 1. Change in mold temperature during the cast of ZSA
- Figure 2. Macrostructure of zinc sacrificial lloys under various conditions of solidification and cooling in-the form with initial temperature of form 20°C(a), 160°C(b) and 310°C(c)
- Figure 3. The scheme of installation of thermocouples for measure the temperature fields of a hardening alloy (1-5) and cast-iron forms (6-8)
- Figure 4. Temperature variation in the solidifying alloys and cast-iron mold at their height at a mold temperature of 20°C (I), 160° C(II), 310°C (III): a, b - cooling and heating curves, respectively; c, d - are the temperature fields in the solidifying alloy and the shape, respectively.
- Figure 5. Change in mass of metal under different cooling conditions at initial temperature of the mold 20(1), 160(2) and 310(3)°C
- Figure 6. Dependence of the cooling time of the casting in the form t1, cooling the mold to the set temperature t2 and the total casting cycle tц from the initial temperature of the mold.
- Figure 7. Influence of the casting temperature on the change of zinc alloys CC by casting into nonmetallic (a) and metallic forms (b)
- Figure 8. Microstructure (x100) of the ZSA1 (I, II, III) and ZSA2 (IV, V, VI) alloys when casting into nonmetallic and metallic (VII, VIII, IX) forms at the following casting temperatures: I, IV, VII - 450 °C; II, V, VII - 500 ° III, VI, IX - 550°C
- Figure 9. Change in CC and microstructure of ZSA1 (a) and ZSA2 (b) alloys depending on the speed of solidification(Vs)
- Figure 10. Construction of a mold for the production of zinc treads
- Figure 11. Changing the temperature of the cast tread when casting into a water-cooled mold 1-5 – the areas, where installed the thermocouples
7. Conclusion:
- It is shown that different cooling conditions lead to a change in the temperature drop across the section of the cast zink sacrificial anodes, changing their electrochemical properties.
- The best conditions for directional solidification of the ingot are ensured at a higher temperature difference (solidification of the alloy in a mold with a temperature not higher than 160°C).
- Investigation of the thermal conditions of solidification of zinc ingots showed that the minimum time for a complete casting cycle, including filling and cooling of the zinc ingot, in the absence of forced cooling is achieved at the initial temperature of the metallic form of 180-200°C.
- Since metal forms are used to produce ingots many times during the shift, the important task of controlling the casting process is to maintain their temperature before the next pouring at a constant level, including using forced cooling by casting into water-cooled shapes with a directed heat sink.
- The present results allow to achieve the highest current capacity and stable potential at anodic polarizations.
8. References:
- [1] D.M. Stefanescu. Science and Engineering of Casting Solidification. 3rd ed. Springer International Publishing AG, Switzerland (2015) p.559.
- [2] J. Dantzig, M. Rappaz. Solidification. Taylor & Francis Group, CRS Press (2009) p.621
- [3] J. Campbell. Casting. 2nd ed. Butterworth-Heinemann, Elsevier (2003) p.335.
- [4] F. S. Yang, F. Ni. Effect of Cooling Rate on the Solidification of Zn-5wt%Al Alloy, Advanced Materials Research (2012) Vol. 366, pp. 502-505.
- [5] Krupiński, M., Krupińska, B., Labisz, K. et al. Influence of cooling rate on crystallization kinetics on microstructure of cast zinc alloys, Journal of Thermal Analysis and Calorimetry (2014), Volume 118, Issue 2, pp 1361–1367.
- [6] R.-N. Ma, Y.-Z. Fan, A. Du, P.-P. Zhang. Effect of cooling rate on morphology and corrosion resistance of Zn-Al-Mg alloy, Cailiao Rechuli Xuebao/Transactions of Materials and Heat Treatment. (2015) Vol. 36(4), pp. 49-55.
- [7] V.A. Kechin, E.Y. Lyublinski. New Sacrificial Anodic Alloys. NACE International, (2018) Paper C2018-11388.
- [8] Chris Jennings,. A comparison of the structure and Consumption Rate for Centrifugally Cast Anodes Compared with Die-Cast Anodes, NACE 2018, Phoenix, USA, Paper C2018-10954.
- [9] Efim Lyublinski, Vladimir Kechin. Formation of basic properties of galvanic anodes during the industrial production, EUROCORR 2017, Prague,Czhech Republic, Paper 72701.
- [10] A. Aghajani, M. Atapour, R. Alibek. Passivation of Zink Anodes in Marine Conditions. Materials Performance, Vol. 55. No. 9, p, 34.
- [11] V. Kechin, E. Lyublinski. New Sacrificial Anodic Alloys, EUROCORR 2016, Montpellier, France, Paper 0-6242.
- [12] V.A. Kechin. Theory and Technology of Cast Sacrificial Materials. Vladimir State University (2004) p. 181.
- [13] V.A. Kechin, E.Y. Lyublinski. Zinc Alloys. Moscow, Metallurgy (1986) p, 247.
- [14] V.A. Kechin , A.B.Kireev. Riser for the production of cast treads sacrificial anodes, Russian Patent Patent №2492020 (2013)
9. Copyright:
- This material is a paper by "Prof. Vladimir Kechin, Prof. Efim Lyublinski, Phd. Evgeny Prusov". Based on "THE ROLE OF THERMAL PROCESSES IN THE FORMATION OF GALVANIC ZINC ANODES PROPERTIES".
- Source of the paper: Not provided in the paper.
This material is summarized based on the above paper, and unauthorized use for commercial purposes is prohibited.
Copyright © 2025 CASTMAN. All rights reserved.